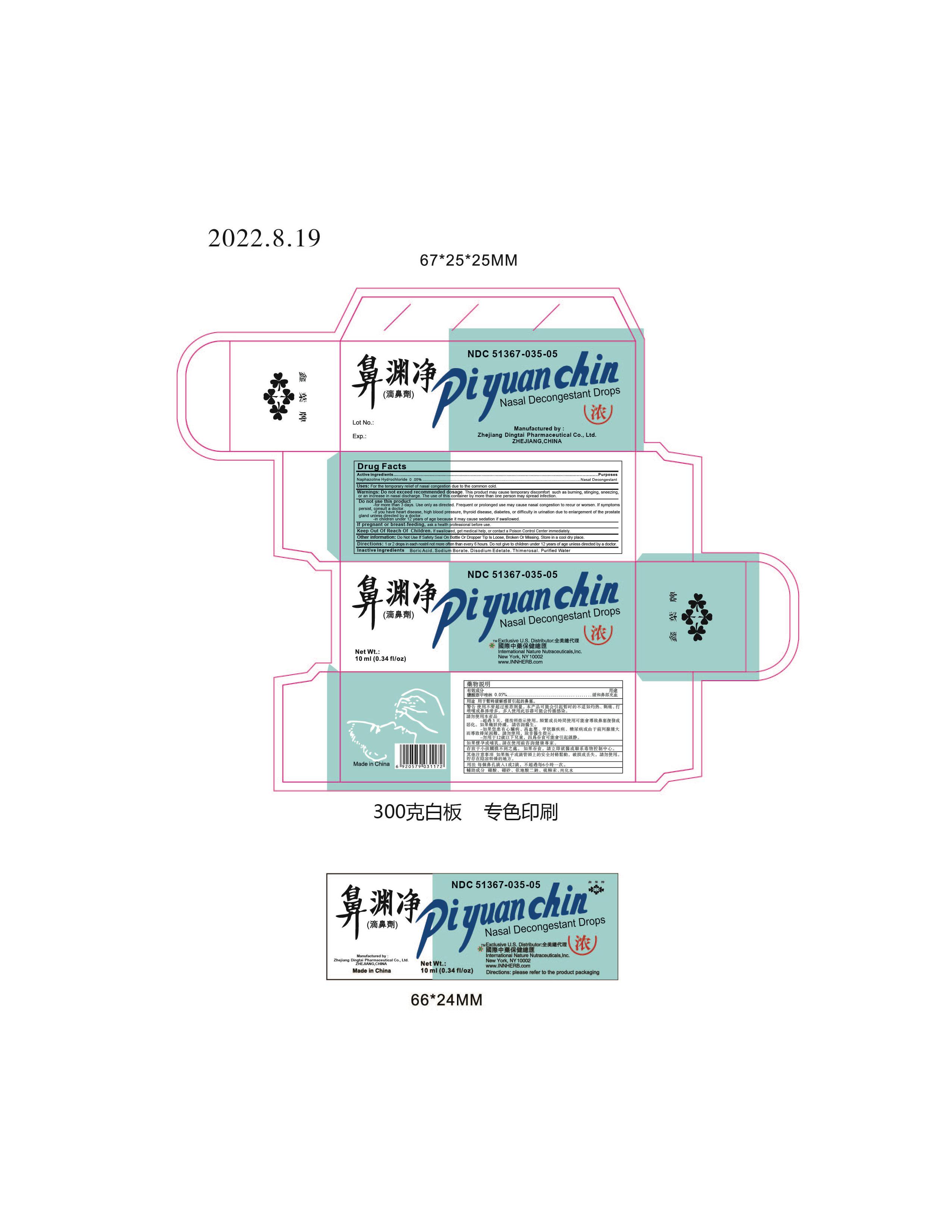
Warnings: Do not exceed recommended dosage.
This product may cause temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge.
The use of this container by more than one person may spread infection.
Do not use this product
- for more than 3 days. Use only as directed. Frequent or prolonged use may cuase nasal congestion to recur or worsen. If symptons persist, consult a doctor.
- If you have heart disease, high blood pressure, thyroid disease, diabetes, or difficulty in urination due to enlargement of the prostate gland unless directed by a doctor.
- in children under 12 years of age because it may cause sedation if swallowed.
Other information:
Do not use if safety seal on bottle or dropper tip is loose, broken or missing. Store in a cool dry place.