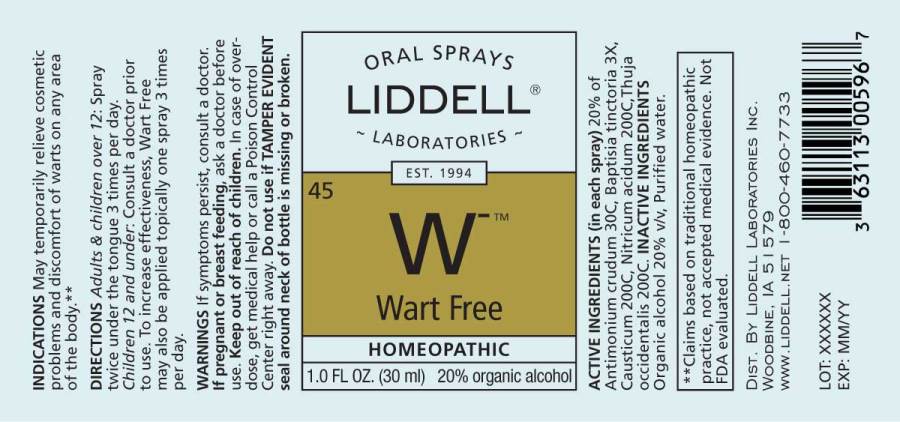
ACTIVE INGREDIENTS:
(in each spray): 20% of Antimonium crudum 30C, Baptisia tinctoria 3X, Causticum 200C, Nitricum acidum 200C, Thuja occidentalis 200C.
INDICATIONS:
May temporarily relieve cosmetic problems and discomfort of warts on any area of the body.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
If symptoms persist, consult a doctor.
If pregnant or breast feeding, ask a doctor before use.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
DIRECTIONS:
Adults and children over 12: Spray twice under the tongue 3 times per day. Children 12 and under: Consult a doctor prior to use.
Wart Free can also be applied topically one spray 3 times per day to increase effectiveness.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.