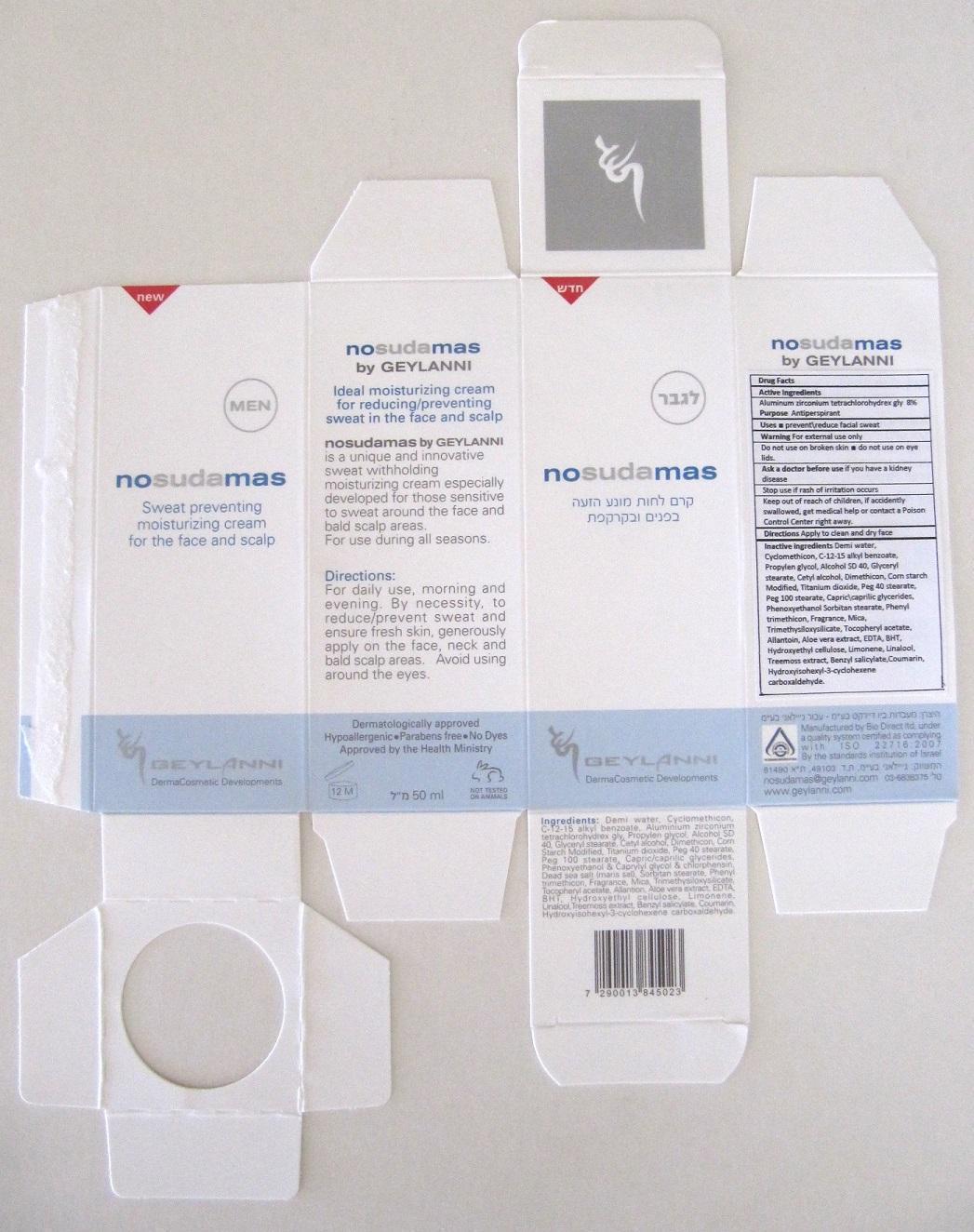
Inactives
WATER
CYCLOMETHICONE
ALKYL (C12-15) BENZOATE
PROPYLENE GLYCOL
ALCOHOL
CETYL ALCOHOL
GLYCERYL MONOSTEARATE
DIMETHICONE
STARCH, CORN
TITANIUM DIOXIDE
PEG-40 STEARATE
PEG-100 STEARATE
PEG-6 CAPRYLIC/CAPRIC GLYCERIDES
PHENOXYETHANOL
SORBITAN MONOSTEARATE
PHENYL TRIMETHICONE
MICA
TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8)
ALPHA-TOCOPHEROL ACETATE
ALLANTOIN
ALOE VERA LEAF
EDETIC ACID
BUTYLATED HYDROXYTOLUENE
HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%)
LIMONENE, (+)-
LINALOOL, (+/-)-
PSEUDEVERNIA FURFURACEA
BENZYL SALICYLATE
COUMARIN
HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE
Directions for Use
Apply to clean and dry face.
Morning and evening by necesity. Generously, apply to clean and dry face.Avoid using around eyes.