Uses
- ▪
- relieves occasional constipation to help restore and maintain regularity
- ▪
- this product generally produces bowel movement in 12 to 72 hours.
Warnings
Choking: Taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting or difficulty in swallowing or breathing after taking this product, seek immediate medical attention.
Ask your doctor before use if you have
- ▪
- abdominal pain, nausea or vomiting
- ▪
- a sudden change in bowel habits that persists over a period of 2 weeks
Ask your doctor or pharmacist before use if
you are taking any other drug. Take this product 2 or more hours before or after other drugs. All laxatives may affect how other drugs work.
When using this product
- ▪
- do not use for more than 7 days unless directed by a doctor
- ▪
- do not take more than 8 caplets in a 24 hour period unless directed by a doctor
Directions
- ▪
- take each dose of this product with at least 8 ounces (a full glass) of water or other fluid. Taking this product without enough liquid may cause choking. See choking warning.
- ▪
- this product works naturally so continued use for one to three days is normally required to provide full benefit. Dosage may vary according to diet, exercise, previous laxative use or severity of constipation
|
|
|
|
|
|
|
|
Other information
- ▪
- each caplet contains: calcium 125 mg
- ▪
- do not use if printed seal under cap is torn or missing
- ▪
- store at 20-25°C (68-77°F)
- ▪
- protect contents from moisture
Inactive ingredients
calcium carbonate, caramel, crospovidone, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, silicon dioxide, sodium lauryl sulfate
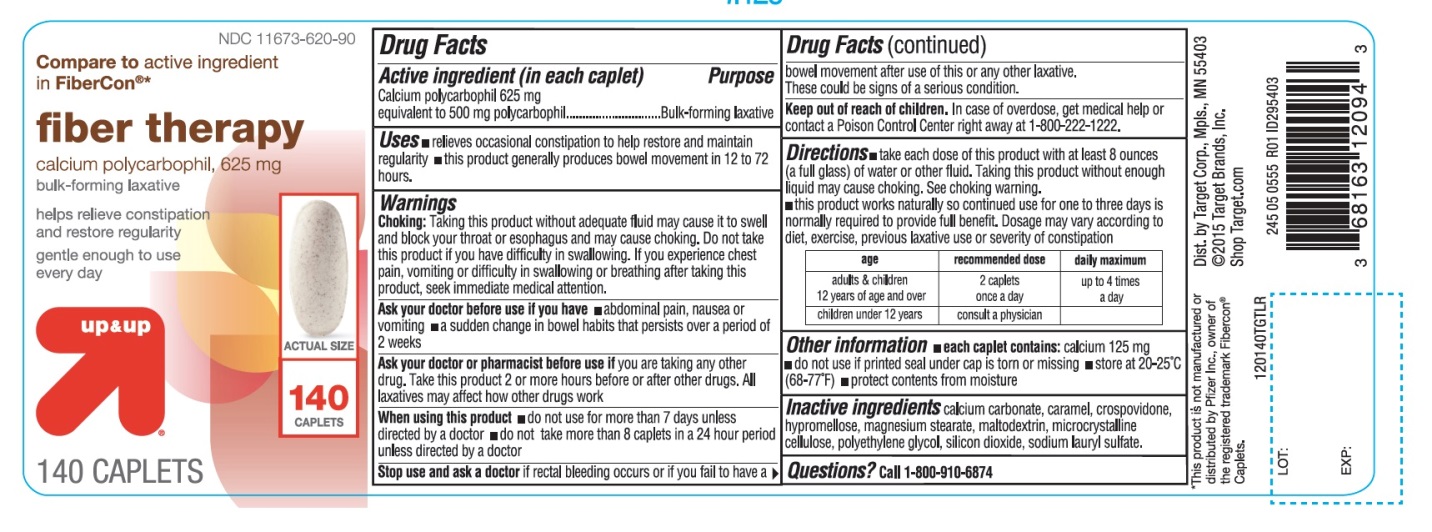
Principal Display Panel
NDC 11673-620-90
Compare to active ingredient in Fibercon® *
fiber therapy
calcium polycarbophil ,625 mg
bulk-forming laxative
helps relieve constipation and restore regularity
gentle enough to use every day
140 CAPLETS
*This product is not manufactured or distributed by Pfizer Inc., owner of the registered trademark Fibercon® Caplets.
245 05 0555 R01 ID295403
Dist.by Target Corp., Mpls., MN 55403
©2015 Target Brands, Inc.
Shop Target.com