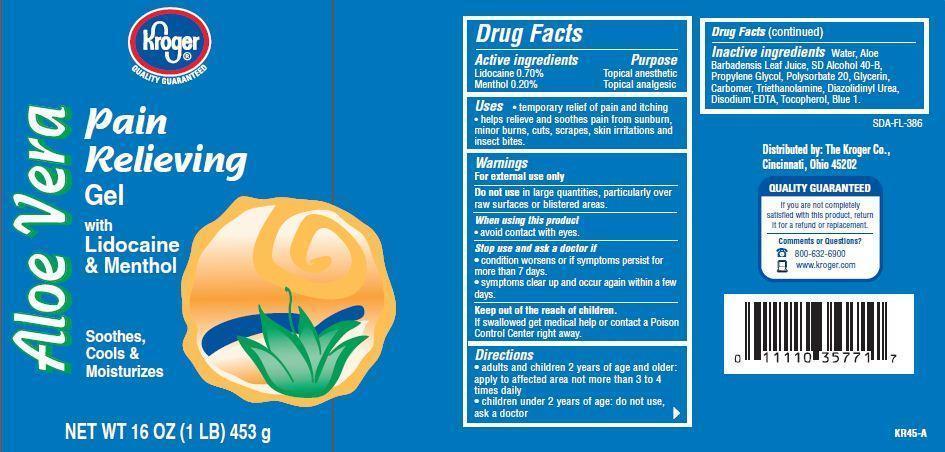
KROGER ALOE VERA PAIN RELIEVING- lidocaine menthol lotion
THE KROGER CO
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Lidocaine 0.70%
Menthol 0.20%
Purpose
Topical anesthetic
Topical analgesic
Uses
- temporary relief of pain and itching
- helps relieve and soothes pain from sunburn, minor burns, cuts, scrapes, skin irritations and insect bites.
Warnings
For external use only
Do not use in large quantities, particularly over raw surfaces or blistered areas.
Stop use and ask a doctor if
- conditions worsens or if symptoms persists for more than 7 days.
- symptoms clear up and occur again within a few days.
Keep out of the reach of children.
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older.apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: do not use, ask a doctor.
Inactive ingredients
Water, Alcohol Denat., Propylene Glycol, Glycerin, Polysorbate 20, Carbomer, Triethanolamine, Aloe Barbadensis Leaf Juice, Disodium EDTA, Tocopherol, Diazolidinyl Urea, Blue 1.
Principal Display Panel
Kroger
QUALITY GURANTEED
Aloe Vera
Pain
Relieving
Gel
with
Lidocaine
and Menthol
Sppthes
Cools and
Moisturizes
NET WT 16 OZ (1 LB) 453 g