CALCIUM CHLORIDE- calcium chloride injection
Amneal-Agila, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
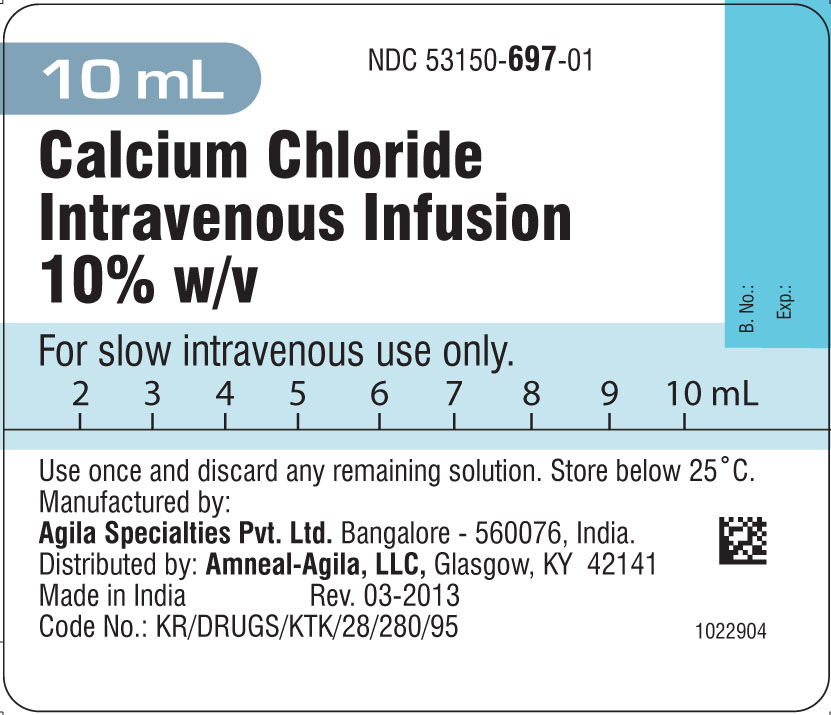
Calcium Chloride Intravenous Infusion
PACKAGE LEAFLET: INFORMATION FOR THE USER
Calcium Chloride Intravenous Infusion 10% w/v
Calcium chloride
|
Read all of this leaflet carefully before you are given this medicine.
|
In this leaflet:
1. What Calcium Chloride Intravenous Infusion 10% w/v is and what it is used for
2. Before Calcium Chloride Intravenous Infusion 10% w/v is given
3. How Calcium Chloride Intravenous Infusion 10% w/v is given
4. Possible side effects
5. How to store Calcium Chloride Intravenous Infusion 10% w/v
6. Further information
1. What Calcium Chloride Intravenous Infusion is and what it is used for
Calcium Chloride is a mineral salt, which is administered to increase the blood levels of Calcium in the body and to get the heart working where potassium levels are too high.
Calcium Chloride is used:
- •
- as part of the resuscitation procedure following a cardiac arrest
- •
- for the treatment of low calcium levels
2. Before Calcium Chloride Intravenous Infusion is given
DO NOT use Calcium Chloride Intravenous Infusion if:
- •
- you are allergic (hypersensitive) to calcium salts or any of the ingredients of Calcium Chloride Intravenous Infusion (see section 6)
- •
- you are taking medicines for heart problems (e.g. digitalis)
- •
- you have low calcium levels due to kidney problems
- •
- you have an excess of calcium present in either your blood or your urine
- •
- you have breathing problems
Take special care with Calcium Chloride Intravenous Infusion if:
- •
- you have kidney problems
- •
- you have heart problems
- •
- you suffer from an inflammatory disorder known as sarcoidosis
If any of the above applies to you or your child, please consult your doctor.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Medicines that may interact with Calcium Chloride Intravenous Infusion include:
- •
- medicines used in the treatment of some bone disordersv (e.g. Paget’s disease)(bisphosphonates) such as didronel or Fosamax
- •
- medicines used to treat bacterial infections (antibiotics) e.g. tetracycline
- •
- medicines used to reduce blood pressure and fluid retention (thiazides) e.g. indapamide
- •
- medicines used to treat heart problems e.g. digoxin
Pregnancy and breast-feeding
If you are pregnant, likely to become pregnant or breast-feeding you must tell your doctor before taking this medicine.
Driving and using machines
There is no known effect of Calcium Chloride Intravenous Infusion on driving or using machines.
3. How Calcium Chloride Intravenous Infusion is given
Your doctor or nurse will administer the injection slowly through a vein (intravenous).
Adults (including the elderly)
- •
- in cases where your heart has stopped a single dose of 10 mL will be given
- •
- if you have recently developed low calcium levels about 3 to 7 mL will be given. This may be repeated as required
Children
- •
- not recommended
If you are given more Calcium Chloride Intravenous Infusion than you should be
As this medicine will be given to you whilst you are in hospital, it is unlikely that you will be given too little or too much, however, tell your doctor or pharmacist if you have any concerns.
4. Possible side effects
Like all medicines, Calcium Chloride Intravenous Infusion can cause side effects, although not everybody gets them.
Possible side effects include:
- •
- A chalky taste in the mouth
- •
- Hot flushes
- •
- Lowered blood pressure
- •
- Loss of appetite
- •
- Feeling sick (nausea)
- •
- Being sick (vomiting)
- •
- Constipation
- •
- Stomach pain
- •
- Feeling weak
- •
- Mental disturbances
- •
- Extreme thirst
- •
- Passing a large amount of urine
- •
- Bone pain
- •
- Calcium deposits in the kidney
- •
- Kidney stones
- •
- Irregular heart beat
- •
- Coma
If any of these side effects get serious, or if you notice any side effects not listed in this leaflet please tell your doctor as soon as possible as this may be a sign of overly calcium blood levels (hypercalcemia).
5. How to store Calcium Chloride Intravenous Infusion
Keep out of the reach and sight of children.
Store below 25°C.
Do not use after expiry date has passed. The doctor or nurse will check that the expiry date on the label has not passed before you are given Calcium Chloride Intravenous Infusion. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicine no longer required. These measures will help protect the environment.
6. Further information
What Calcium Chloride Intravenous Infusion contains
The active ingredient is Calcium Chloride Ph.Eur. (1g)
The other ingredient is Water for injection BP (10 mL).
What Calcium Chloride Intravenous Infusion looks like and the contents of the pack.
The medication is supplied as a single 10 mL prefilled syringe in a plastic box.
(NDC 53150-697-01)
Manufactured by:
Agila Specialties Private Limited,
Bangalore - INDIA.
Distributed by:
Amneal-Agila, LLC
Glasgow, KY 42141
Revision: 03-2013
Healthcare Professional Letter
IMPORTANT DRUG INFORMATION
May XX, 2013
Re: URGENT – 10% Calcium Chloride Injection, USP 10 mL
Unit of Use Syringe Shortage Update
Dear Healthcare Professional,
Due to the current critical shortage of 10% Calcium Chloride Injection, USP 10 mL Unit of Use Syringe in the United States (US) market, Agila Specialties Private Limited (Agila) is coordinating with the Food and Drug Administration (FDA) to increase the availability of 10% Calcium Chloride Injection, USP 10 mL Unit of Use Syringe. In cooperation with the FDA, on behalf of Agila, Amneal-Agila, LLC (Amneal Agila) has initiated temporary importation of a non-FDA approved Calcium Chloride Intravenous Infusion 10% w/w 10 mL Unit of Use Syringe from Agila’s FDA inspected facility in Bangalore, India to the US market.
Amneal-Agila’s Calcium Chloride Intravenous Infusion 10% w/w 10 mL Unit of Use Syringe contains the same active ingredient, Calcium Chloride, in the same strength and concentration as Hospira, Inc.’s FDA approved 10% Calcium Chloride Injection, USP 10 mL Unit of Use Syringe. Each ml of Agila’s product contains 100 mg (1.4 mEq/mL) of Calcium Chloride dihydrate (1.4mEq each of Calcium Ca++ and Cl-) in water for injection.
At this time, no other entity except Amneal-Agila is authorized by the FDA to import or distribute a non-FDA approved Calcium Chloride Intravenous Infusion 10% w/w 10 mL Unit of Use Syringe. Any sales of this product from any other entity other than Amneal-Agila or a distributor or re-seller authorized by Amneal-Agila will be considered in violation of the Federal Food, Drug and Cosmetic Act and may be subject to enforcement by the FDA.
Amneal-Agila’s Calcium Chloride Intravenous Infusion 10% w/w 10 mL Unit of Use Syringe is approved in the United Kingdom and is dispensed with a Medication Guide. The information on the medication guide differs from the full prescribing information for Hospira Inc.’s FDA approved product. For full prescribing information, please refer to the FDA approved package insert for the full prescribing information for Hospira Inc.’s product at:
http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=c4c65e48-85f8-4dcf-6281-06e40959cc79
It is important to note the following Contraindications, Warnings and Precautions in the US FDA
approved package insert for Hospira Inc.’s product:
|
CONTRAINDICATIONS Calcium chloride is contraindicated for cardiac resuscitation in the presence of ventricular fibrillation or in patients with the risk of existing digitalis toxicity. Calcium chloride is not recommended in the treatment of asystole and electromechanical dissociation. |
|
WARNINGS 10% Calcium Chloride Injection, USP is irritating to veins and must not be injected into tissues, since severe necrosis and sloughing may occur. Great care should be taken to avoid extravasation or accidental injection into perivascular tissues. WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum. Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration. |
|
PRECAUTIONS Do not administer unless solution is clear and seal is intact. Discard unused portion. Because of its additive effect, calcium should be administered very cautiously to a patient who is digitalized or who is taking effective doses of digitalis or digitalis-like preparations. Injections should be made slowly through a small needle into a large vein to minimize venous irritation and avoid undesirable reactions. It is particularly important to prevent a high concentration of calcium from reaching the heart because of the danger of cardiac syncope. |
The attached chart highlights the differences in the prescribing information between the FDA-approved Calcium Chloride and Amneal-Agila’s Calcium Chloride Intravenous Infusion 10% w/w 10 mL Unit of Use Syringe.
Please note that the barcode used for Amneal-Agila’s Calcium Chloride Intravenous Infusion 10% w/w 10 mL Unit of Use Syringe may not be appropriately recognized by scanning systems used in the United States. Institutions should confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients.
To order Amneal-Agila’s Calcium Chloride Intravenous Infusion 10% w/w 10 mL Unit of Use Syringe, please contact Customer Service at 1-866-525-7270 or order through the below wholesalers:
AmeriSource Bergen – Order number –
Cardinal Health – Order number –
McKesson Wholesale – Order number –
To report adverse events or medication errors for patients who have used Amneal-Agila’s Calcium Chloride Intravenous Infusion 10% w/w 10 mL Unit of Use Syringe, please contact Drug Safety by telephone at 1-877-835-5472 (Press Option 1) or online @ drugsafety@amneal.com. Adverse reactions may also be reported to the FDA’s MedWatch Adverse Reporting Program either online, by regular mail or fax:
- •
- Online: www.fda.gov/medwatch/report.htm
- •
- Regular Mail: use postage-paid FDA Form 3500 available at www.fda.gov/Medwatch/getforms.htm Mail to: MedWatch, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787
- •
- Fax: 1-800-FDA(332)-0178
Sincerely,
Amneal-Agila Pharmaceuticals
| CALCIUM CHLORIDE
calcium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amneal-Agila, LLC (078578876) |