MAXIMUM STRENGTH GAS RELIEF- simethicone capsule, liquid filled
PURACAP PHARMACEUTICAL LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each softgel)
Simethicone 250 mg
Use
- relieves bloating, pressure or fullness commonly referred to as gas
Warnings
Stop use and ask a doctor if
condition persists
Keep out of reach of children.
Directions
- swallow one or two softgels after a meal
- do not exceed two softgels per 24 hours except under the advice and supervision of a physician
Other information
- store at room temperature 59°-86°F (15°-30°C)
Inactive ingredients
D&C red #33, FD&C blue #1, gelatin, glycerin, purified water, and white edible ink
Questions or Comments?
Call: 1-855-215-8180
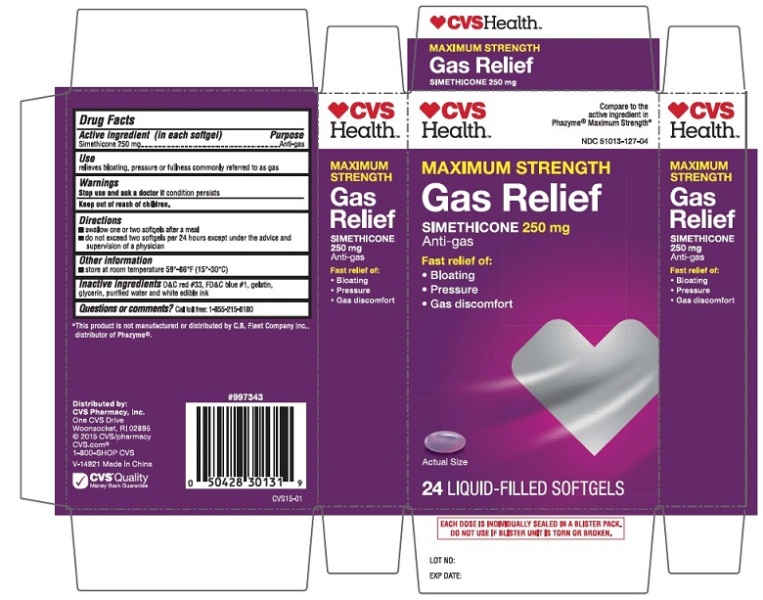
PRINCIPAL DISPLAY PANEL - 24ct
CVS MAXIMUM STRENGTH GAS RELIEF Anti-gas
SIMETHICONE 250 mg
NDC 51013-127-04
Compare to the active ingredient in PHAZYME® Maximum Strength
24 Liquid-Filled SoftGels
PRINCIPAL DISPLAY PANEL - 60ct
CVS MAXIMUM STRENGTH GAS RELIEF Anti-gas
SIMETHICONE 250 mg
NDC 51013-127-22
Compare to the active ingredient in PHAZYME® Maximum Strength
60 Liquid-Filled SoftGels
