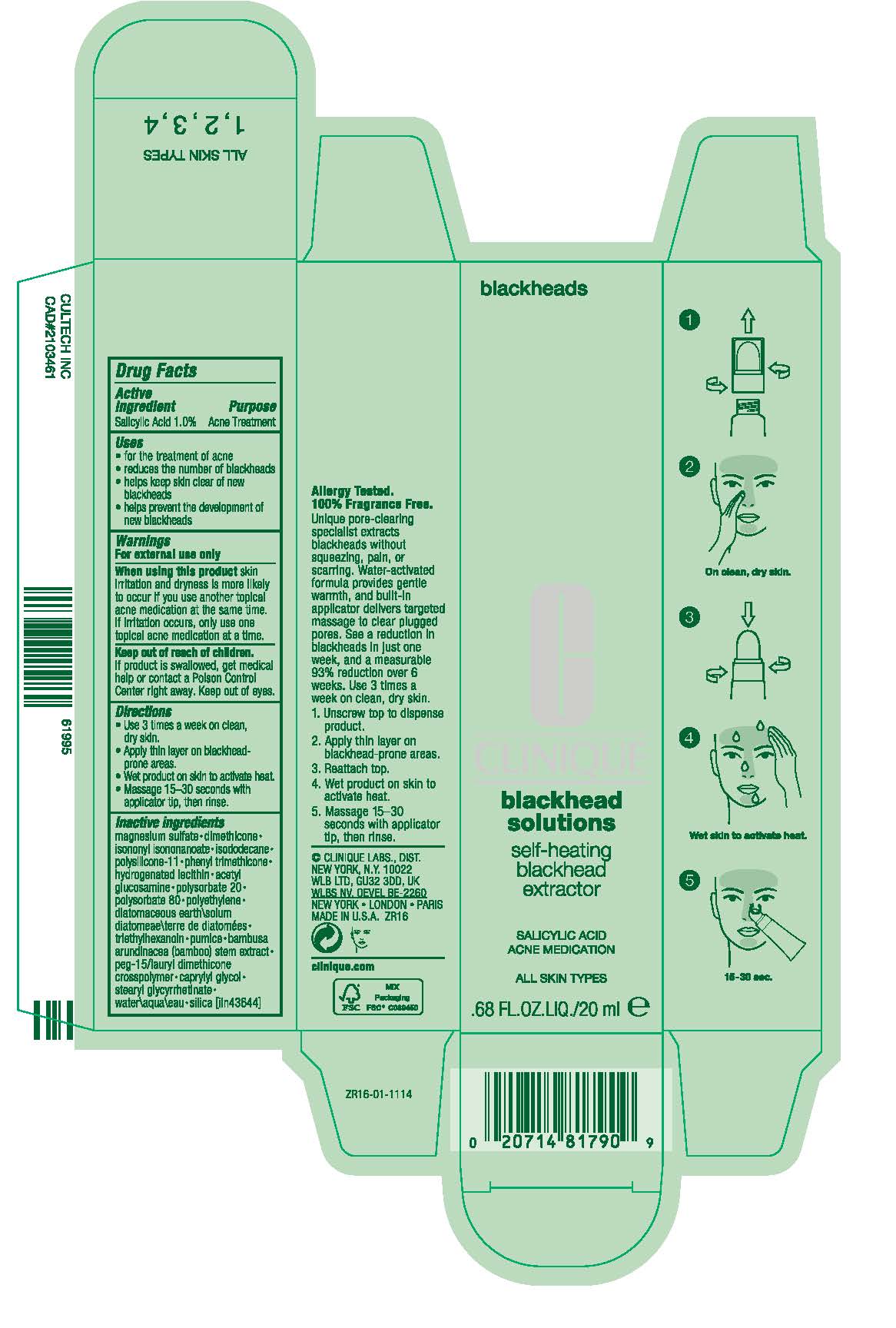
BLACKHEAD SOLUTIONS SELF-HEATING BLACKHEAD EXTRACTOR ACNE MEDICATION- salicylic acid cream
CLINIQUE LABORATORIES LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Salicylic Acid 1.0%
Uses
- reduces the number of blackheads
- helps keep skin clear of new blackheads
- helps prevent development of new blackheads
Warnings
For external use only
When using this product skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only one topical acne medication should be used unless directed by a doctor.
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away. Keep out of eyes.
Directions
- Use 3 times a week on clean, dry skin.
- Unscrew top to dispense product.
- Apply thin layer on blackhead-prone areas.
- Reattach top.
- Wet product on skin to activate heat.
- Massage 15-30 seconds with applicator tip, then rinse.
Inactive ingredients
magnesium sulfate • dimethicone • isononyl isononanoate • isododecane • polysilicone-11 • phenyl trimethicone • hydrogenated lecithin • acetyl glucosamine • polysorbate 20 • polysorbate 80 • polyethylene • diatomaceous earth\solum diatomeae\terre de diatomées • triethylhexanoin • pumice • bambusa arundinacea (bamboo) stem extract • peg-15/lauryl dimethicone crosspolymer • caprylyl glycol • stearyl glycyrrhetinate • water\aqua\eau • silica [iln43644]
PRINCIPAL DISPLAY PANEL - 20 ml Tube Carton
blackheads
CLINIQUE
blackhead
solutions
self-heating
blackhead
extractor
SALICYLIC ACID
ACNE MEDICATION
ALL SKIN TYPES
.68 FL.OZ.LIQ./20 ml e