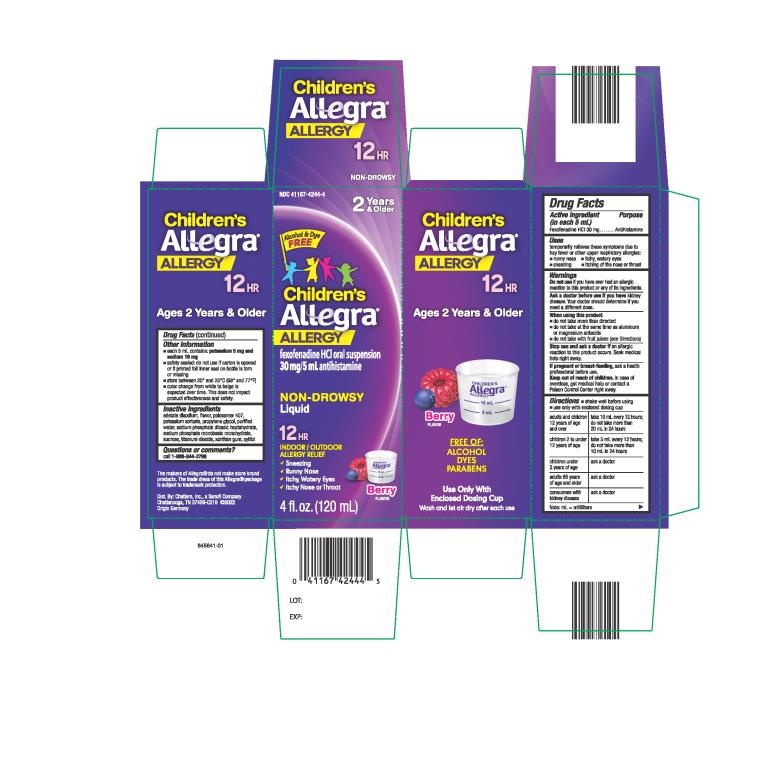
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, water eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
Directions
- shake well before using
- use only with enclosed dosing cup
| adults and children 12 years of age and over | take 10 mL every 12 hours; do not take more than 20 mL in 24 hours |
| children 2 to under 12 years of age | take 5 mL every 12 hours; do not take more than 10 mL in 24 hours |
| children under 2 years of age | ask a doctor |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Note: mL = milliliters
Other information
- each 5 mL contains: each 5 mL contains: potassium 5 mg and sodium 19 mg
- safety sealed: do not use if carton is opened or if printed foil inner seal on bottle is torn or missing
- store between 20° and 25°C (68° and 77°F)
- color change from white to beige is expected over time. This does not impact product effectiveness and safety.
Inactive ingredients
edetate disodium, flavor, poloxamer 407, potassium sorbate, propylene glycol, purified water, sodium phosphate dibasic heptahydrate, sodium phosphate monobasic monohydrate, sucrose, titanium dioxide, xanthan gum, xylitol