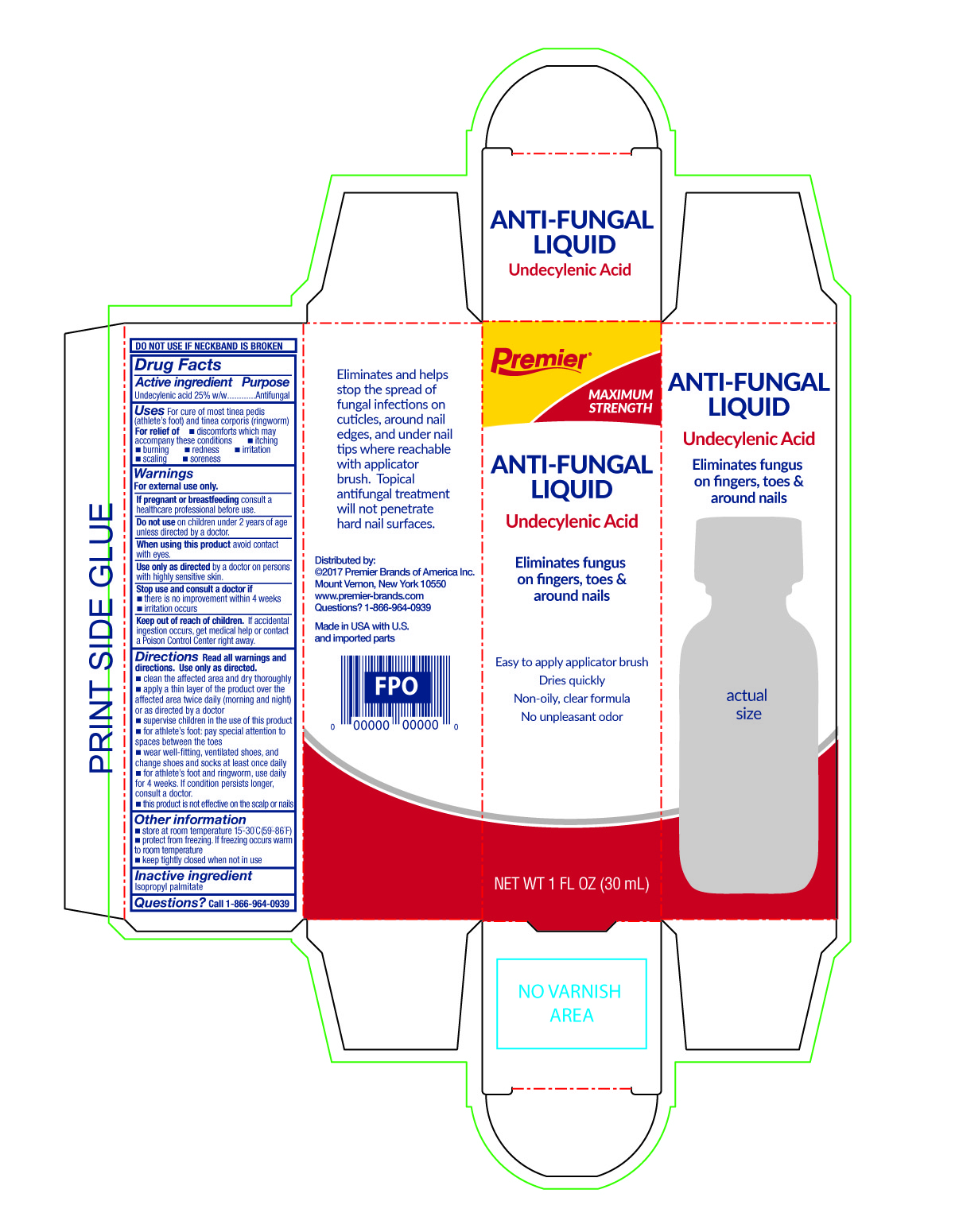
Active ingredient
Undecylenic acid 25%w/w
Uses
For cure of most tinea pedis (athlete's foot) and tinea corporis (ringworm).
For relief of:
- itching
- burning
- redness
- irritation
- scaling
- soreness
- discomfort which may accompany these conditions
Warnings
For external use only
consult a healthcare professional before use.
Do not use
on children 2 years of age unless directed by a doctor.
When using this product
avoid contact with eyes
Use only as directed
by a doctor on persons with highly sensitive skin.
Stop use and consult a doctor if
- there is no improvement within 4 weeks
- irritation occurs
Keep out of children.
If accidental ingestion occurs, get medical help or contact a Poison Control Center right away.
Directions
Read all warnings and directions. Use only as directed.
- clean the affected area and dry thoroughly
- apply a thin layer of the product over the affecter area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between toes
- wear well-fitting, ventilated shoes, and change shoes and socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks. If conditions persists longer, consult a doctor
- this product is not effective on the scalp or nails
Other information
- store at room temperature 15°-30°C (59° - 86°F)
Inactive ingredient
isopropyl palmitate
Questions?
Call 1-866-964-0939
Principal Display Panel
Premier Brands
Maximum Strength
Anti-Fungal Liquid
25% Undecylenic acid
Eliminates fungus on fingers, toes and around nails.
Easy to apply applicator brush
Dries quickly
Non-oily, clear formula
No unpleasant odor
Net Contents: 1 FL OZ (30 mL)

Premier Brands of America Inc.

