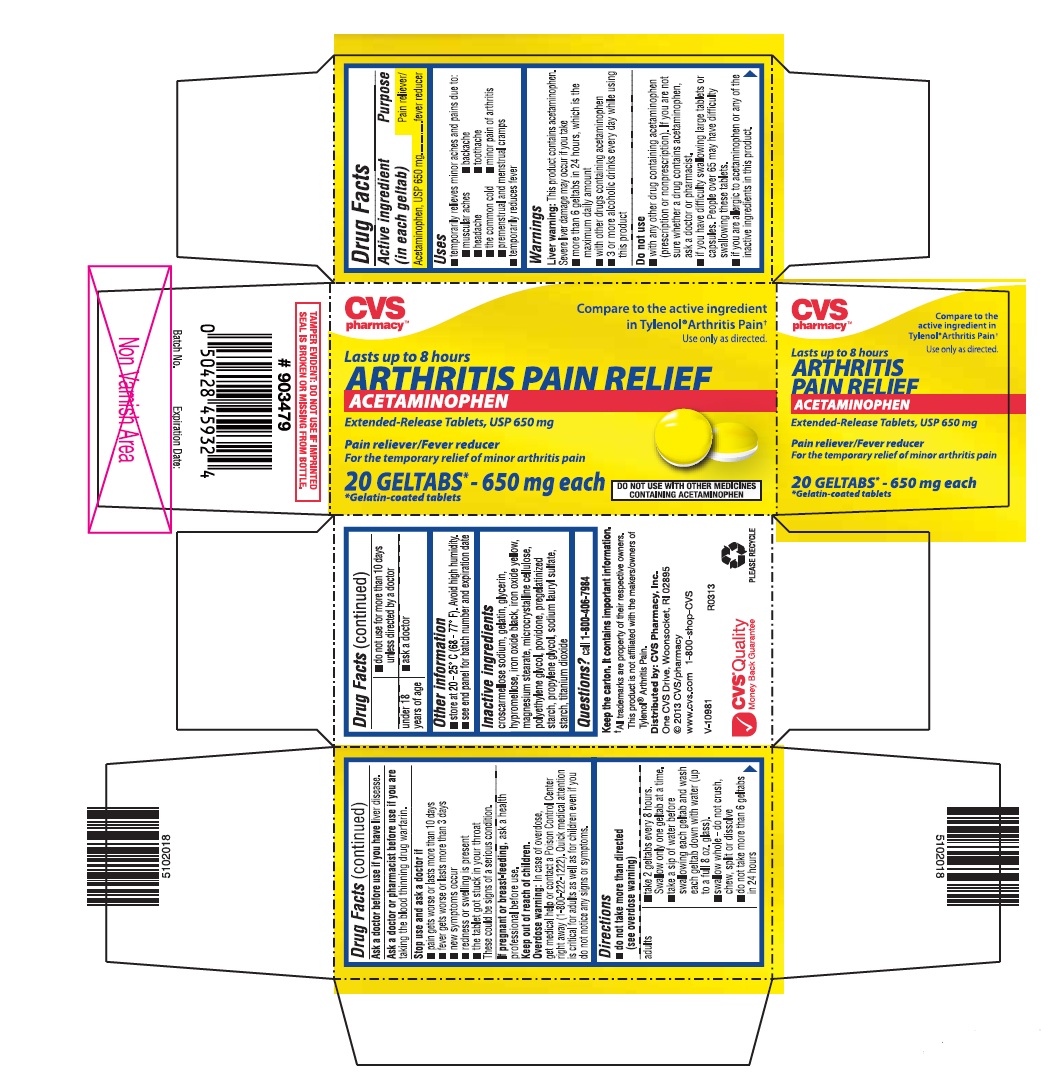
USES
- temporarily relieves minor aches and pains due to:
- muscular aches
- backache
- headache
- toothache
- the common cold
- minor pain of arthritis
- premenstrual and menstrual cramps
- temporarily reduces fever
WARNINGS
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 geltabs in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have difficulty swallowing large tablets or capsules. People over 65 may have difficulty swallowing these tablets.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
DIRECTIONS
-
do not take more than directed (see overdose warning)
adults ▪ take 2 geltabs every 8 hours. Swallow only one geltab at a time.
▪ take a sip of water before swallowing each geltab and wash each geltab down with water (up to a full 8 oz. glass).
▪ swallow whole - do not crush, chew, split or dissolve
▪ do not take more than 6 geltabs in 24 hours
▪ do not use for more than 10 days unless directed by a doctor
under 18 years of age ▪ ask a doctor
OTHER INFORMATION
- store at 20 - 25° C (68 - 77° F). Avoid high humidity.
- see end panel for batch number and expiration date
INACTIVE INGREDIENTS
Croscarmellose sodium, gelatin, glycerin, hypromellose, iron oxide black, iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, propylene glycol, sodium lauryl sulfate, starch, titanium dioxide