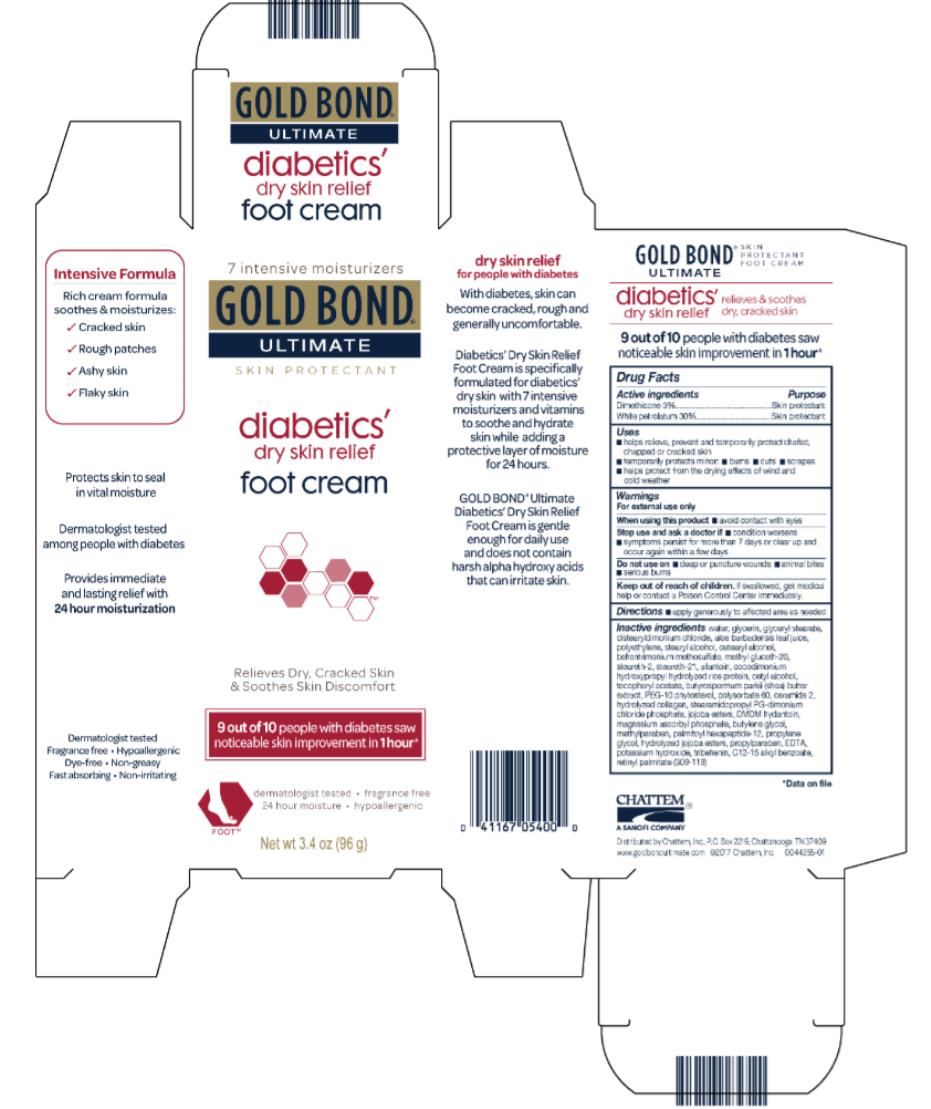
Uses
- helps relieve, prevent and temporarily protect chafed, chapped, cracked or windburned skin
- temporarily protects minor burns, cuts, and scrapes
- helps protect from the drying effects of wind and cold weather
Warnings
For external use only
Inactive ingredients
water, glycerin, glyceryl stearate, distearyldimonium chloride, aloe barbadensis leaf juice, polyethylene, stearyl alcohol, cetearyl alcohol, methyl gluceth-20, behentrimonium methosulfate, steareth-21, steareth-2, allantoin, cocodimonium hydroxypropyl hydrolyzed rice protein, cetyl alcohol, tocopheryl acetate, butyrospermum parkii (shea) butter extract, PEG-10 rapeseed sterol, polysorbate 60, ceramide 2, hydrolyzed collagen, stearamidopropyl PG-dimonium chloride phosphate, jojoba esters, DMDM hydantoin, magnesium ascorbyl phosphate, butylenes glycol, methylparaben, palmitoyl oligopeptide, propylene glycol, hydrolyzed jojoba esters, propylparaben, EDTA, potassium hydroxide, tribehenin, C12-15 alkyl benzoate, retinyl palmitate (309-007)