FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ULTRAVIST® Injection is an iodinated contrast agent indicated for:
1.1 Intra-Arterial Procedures*
ULTRAVIST is indicated for:
- •
- Cerebral arteriography and peripheral arteriography in adults
- •
- Coronary arteriography and left ventriculography, visceral angiography, and aortography in adults
- •
- Radiographic evaluation of cardiac chambers and related arteries in pediatric patients aged 2 years and older
1.2 Intravenous Procedures*
ULTRAVIST is indicated for:
- •
- Excretory urography in adults and pediatric patients aged 2 years and older
- •
- Contrast Computed Tomography (CT) of the head and body (intrathoracic, intra-abdominal, and retroperitoneal regions) for the evaluation of neoplastic and non-neoplastic lesions in adults and pediatric patients aged 2 years and older
- •
- Contrast mammography to visualize known or suspected lesions of the breast in adults, as an adjunct following mammography and/or ultrasound
†Specific concentrations and presentations of ULTRAVIST are recommended for each type of imaging procedure [see Dosage and Administration (2.2, 2.3, 2.4)].
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Information
- •
- ULTRAVIST is for intra-arterial or intravenous use only and must not be administered intrathecally [see Warnings and Precautions (5.1)].
- •
- Specific concentrations and presentations of ULTRAVIST are recommended for each type of imaging procedure [see Dosage and Administrations (2.2, 2.3, 2.4)].
- •
- Hydrate patients, as appropriate, prior to and following the administration of ULTRAVIST [see Warnings and Precautions (5.3)].
- •
- Individualize the volume, concentration, and injection rate of ULTRAVIST according to the specific dosing tables [see Dosage and Administration (2.2, 2.3, 2.4)]. Consider factors such as age, body weight, size of the vessel, and the rate of blood flow within the vessel; also consider extent of opacification required, structure(s) or area to be examined, disease processes affecting the patient, and equipment and technique to be employed.
- •
- Visually inspect ULTRAVIST for particulate matter and/or discoloration, whenever solution and container permit. Do not administer ULTRAVIST if particulate matter (including crystals) and/or discoloration is observed or if containers are defective.
- •
- Use aseptic technique for all handling and administration of ULTRAVIST.
- •
- Warm ULTRAVIST to body temperature before administration.
- •
- ULTRAVIST can be used with 0.9% Sodium Chloride Injection in a power injector suitable for simultaneous injection of contrast [see Dosage and Administration (2.3)]. However, do not mix or inject ULTRAVIST in intravenous administration lines containing other drugs or total nutritional admixtures.
- •
- Discard any unused portion remaining in the single-dose container following initial use.
2.2 Recommended Dosage for Intra-Arterial Procedures in Adults
- •
- The recommended doses for intra-arterial procedures in adults are shown in Table 1.
- •
- Inject at rates approximately equal to the flow rate in the vessel being injected.
|
Imaging Procedure |
Cerebral Arteriography |
Peripheral Arteriography |
Coronary Arteriography and |
Visceral Angiography and Aortography |
|
|
Concentration (mg Iodine per mL) |
300* |
300* |
370* |
370* |
|
|
Intra-Arterial Injection Sites |
Carotid Arteries |
3 mL to 12 mL |
- |
- |
- |
|
Vertebral Arteries |
4 mL to 12 mL |
||||
|
Aortic Arch Injection (four vessel study) |
20 mL to 50 mL |
||||
|
Subclavian or Femoral Artery |
- |
5 mL to 40 mL |
- |
- |
|
|
Aortic Bifurcation (distal runoff) |
25 mL to 50 mL |
||||
|
Right Coronary Artery |
- |
- |
3 mL to 14 mL |
- |
|
|
Left Coronary Artery |
3 mL to 14 mL |
||||
|
Left Ventricle |
30 mL to 60 mL |
||||
|
Aorta and Major Abdominal Branches |
- |
- |
- |
Individualize a volume approximately equal to the blood flow and related to the vascular and pathological characteristics of the specific vessels being studied. |
|
|
Maximum Total Dose |
150 mL |
250 mL |
225 mL |
225 mL |
|
*Use single-dose vials or pharmacy bulk package.
2.3 Recommended Dosage for Intravenous Procedures in Adults
Recommended doses for intravenous procedures in adults are shown in Table 2.
|
Imaging Procedure |
Excretory Urography |
Contrast Computed Tomography |
Contrast Mammography |
|
|
Concentration (mg Iodine per mL) |
300* |
300‡ |
370‡ |
300‡ or 370‡ |
|
Excretory Urography |
1 mL/kg body weight |
- |
- |
- |
|
CT of Head |
- |
50 mL to 200 mL |
41 mL to 162 mL |
- |
|
CT of Body - Single Phase Contrast Bolus Injection Rapid Infusion |
- |
50 mL to 200 mL 100 mL to 200 mL |
41 mL to 162 mL 81 mL to 162 mL |
- |
|
CT of Body – Multiple Phase Contrast |
- |
50 mL to 200 mL total volume Phase 1: 100% contrast, Phase 2: 20% to 60% contrast, using a power injector suitable for simultaneous injection of contrast and 0.9% Sodium Chloride Injection |
41 mL to 162 mL total volume Phase 1: 100% contrast, Phase 2: 20% to 60% contrast, using a power injector suitable for simultaneous injection of contrast and 0.9% Sodium Chloride Injection |
- |
|
Contrast Mammography |
- |
- |
- |
1.5 mL/kg body weight using a power injector at 2 mL/second to 4 mL/second |
|
Maximum Total Dose |
100 mL |
200 mL |
162 mL |
150 mL |
- *Use single-dose vials or pharmacy bulk package.
- ‡Use single-dose vials, pharmacy bulk package or imaging bulk package.
2.4 Recommended Dosage in Pediatric Patients Aged 2 Years and Older
The recommended doses in pediatric patients aged 2 years and older are shown in Table 3.
|
Imaging Procedure |
Intra-arterial |
Intravenous |
|
|
Cardiac Chambers and Related Arteries |
Excretory Urography |
Contrast Computerized Tomography |
|
|
Concentration (mg Iodine/mL) |
370* |
300* |
300‡ |
|
Volume (mL/kg body weight) |
1 to 2 |
1 to 2 |
1 to 2 |
|
Maximum Total Dose (mL/kg) |
4 |
3 |
3 |
- *Use single-dose vials or pharmacy bulk package.
- ‡Use single-dose vials, pharmacy bulk package or imaging bulk package.
2.5 Imaging Instruction for Contrast Mammography
For contrast mammography, use ULTRAVIST with a device that is cleared for dual-energy full field digital mammography.
2.6 Directions for Use of ULTRAVIST Pharmacy Bulk Package
- •
- ULTRAVIST Pharmacy Bulk Package (PBP) is not for direct infusion.
- •
- Perform the transfer of the PBP in a suitable work area, such as a laminar flow hood, utilizing aseptic technique.
- •
- Penetrate the container closure only one time, utilizing a suitable transfer device.
- •
- After initial puncture, use the contents of the PBP within 10 hours. Discard any unused portion
2.7 Directions for Use of ULTRAVIST Imaging Bulk Package
- •
- ULTRAVIST Imaging Bulk Package (IBP) is for intravenous use only.
- •
- ULTRAVIST IBP is for use only with an automated contrast injection system, contrast management system, or contrast media transfer set approved or cleared for use with this contrast agent in this IBP. Please see drug and device labeling for information on devices indicated for use with this IBP and techniques to help assure safe use.
- •
- The IBP is to be used only in a room designated for radiological procedures that involve intravascular administration of a contrast agent.
- •
- Using aseptic technique, penetrate the container closure of the IBP only one time with a suitable sterile component of the automated contrast injection system, contrast management system, or contrast media transfer set approved or cleared for use with this contrast agent in this IBP.
- •
- Once the IBP is punctured, do not remove it from the work area during the entire period of use. Maintain the bottle in an inverted position such that container contents are in continuous contact with the dispensing set.
- •
- After the container closure is punctured, if the integrity of the IBP and the delivery system cannot be assured through direct continuous supervision, discard the IBP and all associated disposables for the automated contrast injection system, contrast management system, or contrast media transfer set.
- •
- A maximum use time from initial puncture is 10 hours. Discard any unused portion remaining in IBP container.
- •
- Storage temperature of IBP after the closure has been entered should not exceed 25°C (77°F) with excursions permitted to 15°C to 30°C (59°F to 86°F); however, it is desirable that the contents be warmed to body temperature prior to injection.
3 DOSAGE FORMS AND STRENGTHS
ULTRAVIST injection is a clear, colorless to slightly yellow, odorless solution available in two concentrations:
300 mg Iodine per mL available as
- •
- 50 mL, 100 mL, 125 mL, and 150 mL in single-dose vials
- •
- 200 mL and 500 mL in pharmacy bulk packages
- •
- 200 mL and 500 mL in imaging bulk packages
370 mg Iodine per mL available as
- •
- 50 mL, 100 mL, 125 mL, and 150 mL in single-dose vials
- •
- 200 mL and 500 mL in pharmacy bulk packages
- •
- 200 mL and 500 mL in imaging bulk packages
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Intrathecal Use
Intrathecal administration, even if inadvertent, can cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. ULTRAVIST is for intra-arterial or intravenous use only [see Dosage and Administration (2.2, 2.3, 2.4)]. ULTRAVIST is not approved for intrathecal use.
5.2 Hypersensitivity Reactions
ULTRAVIST can cause life-threatening or fatal hypersensitivity reactions including anaphylaxis. Manifestations include respiratory arrest, laryngospasm, bronchospasm, angioedema, and shock [see Adverse Reactions (6.2)]. Most severe reactions develop shortly after the start of injection (e.g., within 1 to 3 minutes), but delayed reactions can also occur. There is increased risk of hypersensitivity reactions in patients with a history of previous reaction to a contrast agent and known allergic disorders (that is, bronchial asthma, allergic rhinitis, and food allergies), or other hypersensitivities. Premedication with antihistamines or corticosteroids does not prevent serious life-threatening reactions but may reduce both their incidence and severity.
Obtain a history of allergy, hypersensitivity, or hypersensitivity reactions to iodinated contrast agents and have emergency resuscitation equipment and trained personnel available prior to ULTRAVIST administration. Monitor all patients for hypersensitivity reactions.
5.3 Acute Kidney Injury
Acute kidney injury, including renal failure, may occur after administration of ULTRAVIST. Risk factors include: pre-existing renal insufficiency, dehydration, diabetes mellitus, congestive heart failure, advanced vascular disease, elderly age, concomitant use of nephrotoxic or diuretic medications, multiple myeloma or other paraproteinemia, and repetitive and/or large doses of ULTRAVIST.
Use the lowest necessary dose of ULTRAVIST in patients with renal impairment. Hydrate patients prior to and following ULTRAVIST administration. Do not use laxatives, diuretics, or preparatory dehydration prior to ULTRAVIST administration.
5.4 Cardiovascular Adverse Reactions
ULTRAVIST increases the circulatory osmotic load and may induce acute or delayed hemodynamic disturbances in patients with congestive heart failure, severely impaired renal function, combined renal and hepatic disease, or combined renal and cardiac disease, particularly when repetitive and/or large doses are administered. Fatal cardiovascular reactions have occurred mostly within 10 minutes of ULTRAVIST injection; the main feature was cardiac arrest with cardiovascular disease as the main underlying factor. Hypotensive collapse and shock have occurred. Cardiac decompensation, serious arrhythmias, and myocardial ischemia or infarction can occur during coronary arteriography and ventriculography.
The administration of ULTRAVIST may cause pulmonary edema in patients with heart failure. Based upon published reports, deaths from the administration of iodinated contrast agents range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent). Use the lowest necessary dose of ULTRAVIST in patients with congestive heart failure and always have emergency resuscitation equipment and trained personnel available. Monitor all patients for severe cardiovascular reactions.
5.5 Thromboembolic Events
angiography procedures. During these procedures, increased thrombosis and activation of the complement system can occur. Risk of thromboembolic events can be influenced by: length of procedure, catheter and syringe material, underlying disease state, and concomitant medications.
To decrease thromboembolic events, use meticulous angiographic techniques and minimize the length of the procedure. Avoid blood remaining in contact with syringes containing iodinated contrast agents, which increases the risk of clotting. Avoid angiography in patients with homocystinura because of the risk of inducing thrombosis and embolism.
5.6 Extravasation and Injection Site Reactions
Extravasation can occur with ULTRAVIST, particularly in patients with severe arterial or venous disease. Inflammation, blistering, skin necrosis, and compartment syndrome have been reported following extravasation. In addition, injection site reactions such as pain and swelling at the injection site can also occur [see Adverse Reactions (6.1)]. Ensure intravascular placement of catheters prior to injection. Monitor patients for extravasation and advise patients to seek medical care for progression of symptoms.
5.7 Thyroid Storm in Patients with Hyperthyroidism
Thyroid storm has occurred after the intravascular use of iodinated contrast agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule. Evaluate the risk in such patients before use of ULTRAVIST.
5.8 Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age
Thyroid dysfunction characterized by hypothyroidism or transient thyroid suppression has been reported after both single exposure and multiple exposures to iodinated contrast media (ICM) in pediatric patients 0 to 3 years of age.
Younger age, very low birth weight, prematurity, underlying medical conditions affecting thyroid function, admission to neonatal or pediatric intensive care units, and congenital cardiac conditions are associated with an increased risk of hypothyroidism after ICM exposure. Pediatric patients with congenital cardiac conditions may be at the greatest risk given that they often require high doses of contrast during invasive cardiac procedures.
An underactive thyroid during early life may be harmful for cognitive and neurological development and may require thyroid hormone replacement therapy. After exposure to ICM, individualize thyroid function monitoring based on underlying risk factors, especially in term and preterm neonates.
The safety and effectiveness of ULTRAVIST in pediatric patients younger than 2 years of age have not been established, and ULTRAVIST is not approved for use in pediatric patients younger than 2 years of age [see Use in Specific Populations (8.4)].
5.9 Hypertensive Crisis in Patients with Pheochromocytoma
Hypertensive crisis in patients with pheochromocytoma has occurred with iodinated contrast agents. Closely monitor patients when administering ULTRAVIST if pheochromocytoma or catecholamine-secreting paragangliomas are suspected. Inject the minimum amount of ULTRAVIST necessary and have measures for treatment of a hypertensive crisis readily available.
5.10 Sickle Cell Crisis in Patients with Sickle Cell Disease
Iodinated contrast agents may promote sickling in individuals who are homozygous for sickle cell disease. Hydrate patients prior to and following ULTRAVIST administration and use only if the necessary imaging information cannot be obtained with alternative imaging modalities.
5.11 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering ULTRAVIST to patients with a history of a severe cutaneous adverse reaction to ULTRAVIST.
5.12 Interference with Laboratory Tests
ULTRAVIST can interfere with protein-bound iodine test [see Drug Interactions (7.2)].
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in the labeling:
- •
- Risks Associated with Intrathecal Use [see Warnings and Precautions (5.1)]
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- •
- Acute Kidney Injury [see Warnings and Precautions (5.3)]
- •
- Cardiovascular Adverse Reactions [see Warnings and Precautions (5.4)]
- •
- Thromboembolic Events [see Warnings and Precautions (5.5)]
- •
- Extravasation and Injection Site Reactions [see Warnings and Precautions (5.6)]
- •
- Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age [see Warnings and Precautions (5.8)]
- •
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect or predict the rates observed in practice.
The common adverse reactions reported in >1% of patients in clinical studies with ULTRAVIST are shown in Table 4.
|
Table 4: Adverse Reactions Reported in >1% of Patients Receiving ULTRAVIST in Clinical Trials |
||
|
System Organ Class |
Adverse Reaction |
ULTRAVIST |
|
N=1,142 (%) |
||
|
Nervous system disorders |
Headache |
46 (4) |
|
Dysgeusia |
15 (1.3) |
|
|
Eye disorders |
Abnormal Vision |
12 (1.1) |
|
Cardiac disorders |
Chest pain |
18 (1.6) |
|
Vascular disorders |
Vasodilatation |
30 (2.6) |
|
Gastrointestinal disorders |
Nausea |
42 (3.7) |
|
Vomiting |
22 (1.9) |
|
|
Musculoskeletal and connective tissue disorders |
Back pain |
22 (1.9) |
|
Renal and urinary disorders |
Urinary urgency |
21 (1.8) |
|
General disorders and administration site conditions |
Injection site and infusion site reactions (hemorrhage, hematoma, pain, edema, erythema, rash) |
41 (3.7) |
|
Pain |
13 (1.4) |
|
One or more adverse reactions were recorded in 273 of 1,142 (24%) patients during the clinical trials, coincident with the administration of ULTRAVIST or within the defined duration of the study follow-up period (24–72 hours). ULTRAVIST is often associated with sensations of warmth and/or pain.
Serious, life-threatening, and fatal reactions have been associated with the administration of iodine-containing contrast media, including ULTRAVIST. In clinical trials 7 of 1,142 patients given ULTRAVIST died 5 days or later after drug administration. Also, 10 of 1,142 patients given ULTRAVIST had serious adverse events.
The following adverse reactions were observed in ≤1% of the patients receiving ULTRAVIST:
Cardiac disorders:
atrioventricular block (complete), bradycardia, ventricular extrasystole
Gastrointestinal disorders:
abdominal discomfort, abdominal pain, abdominal pain upper, constipation, diarrhea, dry mouth, dyspepsia, gastrointestinal disorder, gastrointestinal pain, salivation increased, stomach discomfort, rectal tenesmus
General disorders and administration site conditions:
asthenia, chest discomfort, chills, excessive thirst, extravasation, feeling hot, hyperhidrosis, malaise, edema peripheral, pyrexia
Immune system disorders:
asthma, face edema
Investigations:
blood lactate dehydrogenase increased, blood urea increased, hemoglobin increased, white blood cell count increased
Musculoskeletal and connective tissue disorders:
arthralgia, musculoskeletal pain, myasthenia, neck pain, pain in extremity
Nervous system disorders:
agitation, confusion, convulsion, dizziness, hypertonia, hypesthesia, incoordination, neuropathy, somnolence, speech disorder, tremor, paresthesia, visual field defect
Psychiatric disorders:
anxiety
Renal and urinary disorders:
dysuria, renal pain, urinary retention
Respiratory, thoracic and mediastinal disorders:
apnea, cough increased, dyspnea, hypoxia, pharyngeal edema, pharyngitis, pleural effusion, pulmonary hypertension, respiratory disorder, sore throat
Skin and subcutaneous tissue disorders:
erythema, pruritus, rash, urticaria
Vascular disorders:
coronary artery thrombosis, flushing, hypertension, hypotension, peripheral vascular disorder, syncope, vascular anomaly
Pediatric Patients
A total of 274 pediatric patients were evaluated with intra-arterial coronary angiography (n=60), intravenous contrast computerized tomography (CT) (n=87), excretory urography (n=99), and 28 other procedures. These patients received 1 mL/kg to 2 mL/kg body weight of a concentration of 300 mg Iodine per mL for intravenous contrast CT or excretory urography and 370 mg Iodine per mL for intra-arterial and intracardiac administration in the radiographic evaluation of the heart cavities and major arteries [see Dosage and Administration (2.4)]. Among these, 131 were 2 to 12 years old, 57 were adolescents, and 86 were unreported or other ages. There were 148 females, 94 males, and 32 in whom gender was not reported. The racial distribution was: White 33.9%, Black 0.4%, Asian 2.2%, and unknown 63.5%. The overall character, quality, and severity of adverse reactions in pediatric patients are generally similar to those reported in adult patients.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ULTRAVIST. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Adverse reactions reported in foreign postmarketing surveillance and other trials with the use of ULTRAVIST include:
Cardiac disorders:
cardiac arrest, ventricular fibrillation, atrial fibrillation, tachycardia, palpitations, congestive heart failure, myocardial infarction, angina pectoris
Ear and labyrinth disorders:
vertigo, tinnitus
Endocrine disorders:
hyperthyroidism, thyrotoxic crisis, hypothyroidism
Eye disorders:
mydriasis, lacrimation disorder
Gastrointestinal disorders:
dysphagia, swelling of salivary glands
Immune system disorders:
anaphylactoid reaction (including fatal cases), respiratory arrest, anaphylactoid shock, angioedema, laryngeal edema, laryngospasm, bronchospasm, hypersensitivity
Musculoskeletal and connective tissue disorders:
compartment syndrome in case of extravasation
Nervous system disorders:
cerebral ischemia/infarction, paralysis, paresis, transient cortical blindness, aphasia, coma, unconsciousness, amnesia, hypotonia, aggravation of myasthenia gravis symptoms
Renal and urinary disorders:
renal failure, hematuria
Respiratory, thoracic and mediastinal disorders:
pulmonary edema, acute respiratory distress syndrome, asthma
Skin and subcutaneous tissue disorders:
Reactions range from mild (e.g., rash, erythema, pruritus, urticaria and skin discoloration) to severe [e.g., Stevens-Johnson Syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms (DRESS)].
Vascular disorders:
vasospasm
Pediatric Patients
Additional adverse reactions reported in pediatric patients from foreign marketing surveillance or other information include: epistaxis, migraine, joint disorder (effusion), muscle cramps, mucous membrane disorder (mucosal swelling), conjunctivitis, fixed eruptions, diabetes insipidus, and brain edema.
6.3 Pediatrics
The overall character, quality, and severity of adverse reactions in pediatric patients are generally similar to those reported in adult patients. Additional adverse reactions reported in pediatric patients from foreign marketing surveillance or other information are: epistaxis, angioedema, migraine, joint disorder (effusion), muscle cramps, mucous membrane disorder (mucosal swelling), conjunctivitis, hypoxia, fixed eruptions, vertigo, diabetes insipidus, and brain edema [see Use in Specific Populations (8.4)].
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
Metformin
In patients with renal impairment, biguanides can cause lactic acidosis. Iodinated contrast agents appear to increase the risk of metformin-induced lactic acidosis, possibly as a result of worsening renal function. Stop metformin at the time of, or prior to, ULTRAVIST administration in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will be administered intra-arterial iodinated contrast agents. Re-evaluate eGFR 48 hours after the imaging procedure and reinstitute only after renal function is stable.
7.2 Drug-Laboratory Test Interactions
Protein-Bound Iodine Test
Iodinated contrast agents, including ULTRAVIST, will temporarily increase protein-bound iodine in blood. Do not perform protein-bound iodine test for at least 16 days following administration of ULTRAVIST. However, thyroid function tests which do not depend on iodine estimations, for example, T3 resin uptake and total or free thyroxine (T4) assays are not affected.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data on ULTRAVIST use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Iopromide crosses the placenta and reaches fetal tissues in small amounts (see Data). In animal reproduction studies, intravenous administration of iopromide to pregnant rats and rabbits during organogenesis at doses up to0.35 and 0.7 times, respectively, the maximum recommended human dose based on body surface area resulted in no relevant adverse developmental effects (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Limited case reports demonstrate that intravenously administered iodinated contrast agents, including iopromide, cross the placenta and are visualized in the digestive tract of exposed infants after birth.
Animal Data
Reproduction studies were performed with intravenous iopromide in rats (day 6 to 15 of gestation) and rabbits (day 6 to 18 of gestation) at dose levels of 0, 0.37, 1.11, and 3.7 g iodine per kg, corresponding to doses up to 0.35 times (rats) and 0.7 times (rabbits) the maximum human recommended dose based on body surface area. Iopromide was not teratogenic at any dose level in rats and rabbits and embryolethality was observed in rabbits that received 3.7 g iodine per kg, but this was considered to have been secondary to maternal toxicity.
8.2 Lactation
Risk Summary
There are no data on the presence of iopromide in human milk, the effects on the breastfed infant, or the effects on milk production. Iodinated contrast agents are poorly excreted into human milk and are poorly absorbed by the gastrointestinal tract of a breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ULTRAVIST and any potential adverse effects on the breastfed infant from ULTRAVIST or from the underlying maternal condition (see Clinical Considerations).
Clinical Considerations
Interruption of breastfeeding after exposure to iodinated contrast agents is not necessary because the potential exposure of the breastfed infant to iodine is small. However, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 12 to 24 hours (approximately 5 elimination half-lives) after ULTRAVIST administration in order to minimize drug exposure to a breast fed infant.
8.4 Pediatric Use
The safety and efficacy of ULTRAVIST have been established in pediatric patients aged 2 years and older for radiographic evaluation of cardiac chambers and related arteries, excretory urography, and contrast computed tomography of head and body. Use of ULTRAVIST in these age groups for these indications is supported by evidence from adequate and well-controlled studies in adults and additional safety data in pediatric patients aged 2 years and older, including data from published studies [see Adverse Reactions (6.1, 6.2) and Clinical Studies (14.1, 14.2)].
Pediatric patients at higher risk of experiencing an adverse reaction during and after administration of any contrast agent include those with asthma, sensitivity to medication and/or allergens, cyanotic and acyanotic heart disease, congestive heart failure, or serum creatinine greater than 1.5 mg/dL.
Thyroid function tests indicative of thyroid dysfunction, characterized by hypothyroidism or transient thyroid suppression have been reported following iodinated contrast media administration in pediatric patients, including term and preterm neonates; Some patients were treated for hypothyroidism. After exposure to iodinated contrast media, individualize thyroid function monitoring in pediatric patients 0 to 3 years of age based on underlying risk factors, especially in term and preterm neonates [see Warnings and Precautions (5.8) and Adverse Reactions (6.2)].
Safety and effectiveness of ULTRAVIST have not been established in pediatric patients younger than 2 years for radiographic evaluation of cardiac chambers and related arteries, excretory urography, and contrast computed tomography of head and body.
Safety and effectiveness of ULTRAVIST for cerebral arteriography, peripheral arteriography, coronary arteriography and left ventriculography, visceral angiography, aortography, and contrast mammography have not been established in pediatric patients.
8.5 Geriatric Use
In a clinical study of ULTRAVIST for CT, 96/434 (22.1%) of patients were 65 and over. No overall differences in safety were observed between these patients and younger patients. Other reported clinical experience has not identified differences in response between the elderly and younger patients. Iopromide is known to be substantially excreted by the kidney, and the risk of adverse reactions to iopromide may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
The clearance of iopromide decreases with increasing degree of renal impairment and results in delayed opacification of the urinary system [see Clinical Pharmacology (12.3)]. In addition, preexisting renal impairment increases the risk for acute kidney injury [see Warnings and Precautions (5.3)]. Iopromide can be removed by dialysis.
10 OVERDOSAGE
The manifestations of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular systems. Treatment of an overdosage is directed toward the support of all vital functions, and prompt institution of symptomatic therapy. Iopromide can be removed by dialysis.
11 DESCRIPTION
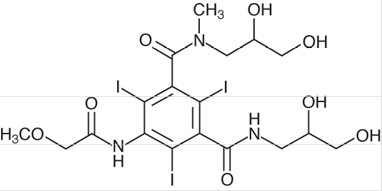
ULTRAVIST(iopromide) injection is a nonionic radiographic contrast agent for intra-arterial or intravenous administration. The chemical name for iopromide is N,N'-Bis(2,3-dihydroxypropyl)-2,4,6-triiodo-5-[(methoxyacetyl)amino]-N-methyl- 1,3- benzenedicarboxamide. Iopromide has a molecular weight of 791.12 (iodine content 48.12%).
Iopromide has the following structural formula:
Each mL contains 623.4 mg or 768.86 mg iopromide (300 mg or 370 mg iodine, respectively) and the following inactive ingredients: 0.1 mg edetate calcium disodium as a stabilizer and 2.42 mg tromethamine as a buffer. It may also contain sodium hydroxide or hydrochloric acid to adjust pH to 7.4 (6.5–8) at 25± 2°C and contains no preservatives.
ULTRAVIST is a sterile (sterilized by autoclaving), clear, colorless to slightly yellow, odorless, pyrogen-free aqueous solution and has the following physicochemical properties:
|
Property |
Concentration of ULTRAVIST (mg Iodine/mL) |
||
|
300 |
370 |
||
|
Osmolality*(mOsmol/kg water) |
@ 37°C |
607 |
774 |
|
Osmolarity*(mOsmol/L) |
@ 37°C |
428 |
496 |
|
Viscosity (cP) |
@ 20°C @ 37°C |
9.2 4.9 |
22 10 |
|
Density (g/mL) |
@ 20°C @ 37°C |
1.330 1.322 |
1.409 1.399 |
- *Osmolality was measured by vapor-pressure osmometry. Osmolarity was calculated from the measured osmolal concentrations.
ULTRAVIST 300 mg Iodine per mL and 370 mg Iodine per mL have osmolalities respectively 2.1 and 2.7 times that of plasma (285 mOsmol/kg water).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Intravascular injection of iopromide opacifies those vessels where the contrast agent is present, permitting radiographic visualization of the internal structures through attenuation of photons. In imaging of the body, iodinated contrast agents diffuse from the vessels into the extravascular space. In normal brain with an intact blood-brain barrier, contrast does not diffuse into the extravascular space. In patients with a disrupted blood-brain barrier, contrast agent accumulates in the extravascular space in the region of disruption.
12.2 Pharmacodynamics
Following ULTRAVIST administration, the degree of contrast enhancement is related to the iodine concentration in the tissue of interest.
12.3 Pharmacokinetics
Distribution
After intravenous administration to healthy adult subjects, plasma iopromide concentration time profile shows an initial distribution phase with a half-life of 0.24 hour; a main elimination phase with a half-life of 2 hours; and a terminal elimination phase with a half-life of 6.2 hours. The total volume of distribution at steady state is about 16 L suggesting distribution into extracellular space. Plasma protein binding of iopromide is 1%.
Iodinated contrast agents cross a disrupted blood-brain barrier.
Elimination
Excretion
The amounts excreted unchanged in urine represent 97% of the dose in adult healthy subjects. Only 2% of the dose is recovered in the feces. Similar recoveries in urine and feces are observed in middle-aged and elderly patients. This finding suggests that, compared to the renal route, biliary and/or gastrointestinal excretion is not important for iopromide. During the slower terminal phase, only 3% of the dose is eliminated; 97% of the dose is disposed of during the earlier phases, the largest part of which occurs during the main elimination phase. The ratio of the renal clearance of iopromide to the creatinine clearance is 0.82 suggesting that iopromide is mainly excreted by glomerular filtration. Additional tubular reabsorption is possible. Pharmacokinetics of iopromide at intravenous doses up to 80 g iodine are dose proportionate and first order.
The mean total and renal clearances are 107 mL/min and 104 mL/min, respectively.
Specific Populations
Age
Middle-aged (49 to 64 years) and elderly patients (65 to 70 years), without significantly impaired renal function, who received ULTRAVIST in doses corresponding to 9–30 g iodine, had mean steady-state volumes of distribution that ranged between 30–40 L. Mean total and renal clearances were between 81–125 mL/min and 70–115 mL/min, respectively, in these patients, and were similar to the values found in the young subjects. The distribution phase half-life in this patient population was 0.1 hour, the main elimination phase half-life was 2.3 hours, and the terminal elimination phase half-life was 40 hours. The urinary excretion (97% of the dose) and fecal excretion (2%) were comparable to that observed in young healthy subjects, suggesting that, compared to the renal route, biliary and/or gastrointestinal excretion is not significant for iopromide.
Patients with Renal Impairment
A pharmacokinetic study in patients with mild (n=2), moderate (n=6), and severe (n=3) renal impairment was conducted. Mild and moderate patients had a CLCR between 30 to 80 mL/min/1.73 m2. Severe patients had a CLCR between 10 to 30 mL/min/1.73 m2. ULTRAVIST 300 mg Iodine per mL was administered intravenously using a single-dose bolus injection. The total clearance of iopromide was decreased proportionately to the baseline decrease in creatinine clearance. The plasma AUC increased about 2-fold in patients with moderate renal impairment and 6-fold in patients with severe renal impairment compared to subjects with normal renal function. The terminal half-life increased from 2.2 hours for subjects with normal renal function to 11.6 hours in patients with severe renal impairment. The peak plasma concentration of iopromide was not influenced by the extent of renal impairment [see Use in Specific Populations (8.6)].
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed with iopromide to evaluate carcinogenic potential or effects on fertility. Iopromide was not genotoxic in a series of studies including the Ames test, an in vitro human lymphocytes analysis of chromosomal aberrations, an in vivo mouse micro-nucleus assay, and in an in vivo mouse dominant lethal assay.
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies
The following intra-arterial and intravenous procedures were studied with one of two concentrations of ULTRAVIST (300 mg Iodine per mL and 370 mg Iodine per mL): aortography/visceral angiography, coronary arteriography and left ventriculography, cerebral arteriography, peripheral arteriography, contrast computed tomography (CT) of head and body, excretory urography, and contrast mammography.
Efficacy assessment for most indications was based on the global evaluation of the quality of the images by rating visualization as either excellent, good, poor, or no image, and on the ability to make a diagnosis. For contrast mammography, efficacy assessment was based on rating visualized contrast uptake as negative, none, moderate, or intense.
14.2 Intra-Arterial Studies
Cerebral arteriography was evaluated in two randomized, double-blind clinical trials of ULTRAVIST 300 mg Iodine per mL in 80 patients with conditions such as altered cerebrovascular perfusion and/or permeability occurring in central nervous system diseases due to various CNS disorders. Visualization ratings were good or excellent in 99% of the patients with ULTRAVIST; a radiologic diagnosis was made in the majority of the patients. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
Coronary arteriography/left ventriculography was evaluated in two randomized, double-blind clinical trials and one unblinded, unrandomized clinical trial of ULTRAVIST 370 mg Iodine per mL in 106 patients with conditions such as altered coronary artery perfusion due to metabolic causes and in patients with conditions such as altered ventricular function. Visualization ratings were good or excellent in 99% or more of the patients with ULTRAVIST; a radiologic diagnosis was made in the majority of the patients. Confirmation of the radiologic findings by other diagnostic methods was not obtained.
Aortography/visceral angiography was evaluated in two randomized, double-blind clinical trials of ULTRAVIST 370 mg Iodine per mL in 78 patients with conditions such as altered aortic blood flow and/or visceral vascular disorders. Visualization ratings were good or excellent in the majority of the patients with ULTRAVIST; a radiologic diagnosis was made in 99% of the patients. Confirmation of radiologic findings by other diagnostic methods was not obtained. The risks of renal arteriography could not be analyzed.
Similar studies were completed with comparable findings noted in peripheral arteriography.
14.3 Intravenous Studies
Contrast CT of head and body was evaluated in three randomized, double-blind clinical trials of ULTRAVIST 300 mg Iodine per mL in 95 patients with vascular disorders. Visualization ratings were good or excellent in 99% of the patients with ULTRAVIST; a radiologic diagnosis was made in the majority of the patients. Confirmation of contrast CT findings by other diagnostic methods was not obtained.
ULTRAVIST was evaluated in a blinded reader trial for CT of the head and body. Among the 382 patients who were evaluated with ULTRAVIST 370 mg Iodine per mL, visualization ratings were good or excellent in approximately 97% of patients.
Similar studies were completed with comparable findings noted in excretory urography.
Contrast mammography was evaluated in a published prospective study of 216 women (age range 26–85 years; mean age 54.6 years) who had BI-RADS 3, 4, or 5 findings on mammography and were evaluated using ULTRAVIST 300 mg Iodine per mL. Patients with breast implants, pregnancy or possible pregnancy, history of hypersensitivity reaction to iodinated contrast agents, and renal insufficiency were excluded from study. All breast lesions were evaluated in a blinded manner. A total of 226 lesions was evaluated, including 98 (43%) malignant lesions. Visualization of lesion contrast was scored on a 4-point scale, with 4 (2%) lesions rated as below background (negative), 93 (41%) as none, 46 (20%) as moderate, and 83 (37%) as intense.
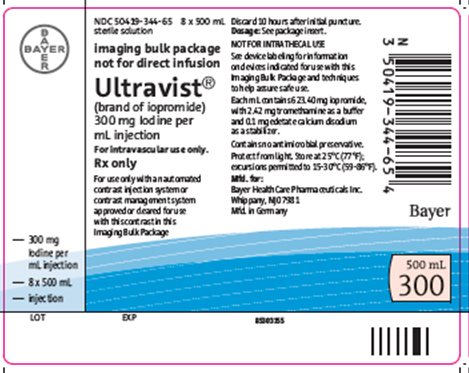
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ULTRAVIST injection is a sterile, clear, colorless to slightly yellow, odorless, pyrogen-free aqueous solution available in the following presentations:
|
ULTRAVIST 300 mg Iodine per mL |
|||
|
Package Type |
Volume |
Sale Unit |
NDC |
|
Single-Dose Vials |
50 mL |
Carton of 10 |
50419-344-05 |
|
100 mL |
Carton of 10 |
50419-344-10 |
|
|
125 mL |
Carton of 10 |
50419-344-12 |
|
|
150 mL |
Carton of 10 |
50419-344-15 |
|
|
Pharmacy Bulk Package |
200 mL |
Carton of 10 |
50419-344-21 |
|
500 mL |
Carton of 8 |
50419-344-58 |
|
|
Imaging Bulk Package |
200 mL |
Carton of 10 |
50419-344-23 |
|
500 mL |
Carton of 8 |
50419-344-65 |
|
|
ULTRAVIST 370 mg Iodine per mL |
|||
|
Package Type |
Volume |
Sale Unit |
NDC |
|
Single-Dose Vials |
50 mL |
Carton of 10 |
50419-346-05 |
|
100 mL |
Carton of 10 |
50419-346-10 |
|
|
150 mL |
Carton of 10 |
50419-346-15 |
|
|
200 mL |
Carton of 10 |
50419-346-20 |
|
|
Pharmacy Bulk Package |
200 mL |
Carton of 10 |
50419-346-26 |
|
500 mL |
Carton of 8 |
50419-346-58 |
|
|
Imaging Bulk Package |
200 mL |
Carton of 10 |
50419-346-28 |
|
500 mL |
Carton of 8 |
50419-346-65 |
|
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise the patient concerning the risk of hypersensitivity reactions that can occur both during and after ULTRAVIST administration. Advise the patient to report any signs or symptoms of hypersensitivity reactions during the procedure and to seek immediate medical attention for any signs or symptoms experienced after discharge [see Warnings and Precautions (5.2)].
Advise patients to inform their physician if they develop a rash after receiving ULTRAVIST [see Warnings and Precautions (5.11)]
Acute Kidney Injury
Advise the patient concerning appropriate hydration to decrease the risk of contrast induced kidney injury [see Warnings and Precautions (5.3)].
Extravasation
If extravasation occurs during injection, advise patients to seek medical care for progression of symptoms [see Warnings and Precautions (5.6)].
Thyroid Dysfunction
Advise parents/caregivers about the risk of developing thyroid dysfunction after ULTRAVIST administration. Advise parents/caregivers about when to seek medical care for their child to monitor for thyroid function [see Warnings and Precautions (5.8)].
Lactation
Advise lactating women that interruption of breast feeding is not necessary, however, to avoid any exposure a lactating woman may consider pumping and discarding breast milk for 12 to 24 hours after ULTRAVIST administration [see Use in Specific Populations (8.2)].
Manufactured for:
- Bayer HealthCare Pharmaceuticals Inc.
Whippany, NJ 07981 - Manufactured in Germany
©1995, Bayer HealthCare Pharmaceuticals Inc. All rights reserved.
The following are representative examples of ULTRAVIST labeling. See the "How Supplied" section for a complete listing of all components.
Package/Label Display Panel 240mg Iodine per 50mL
NDC 50419-342-05 50 mL
sterile solution
Ultravist®
(brand of iopromide)
240 mgI/mL injection
Rx only
Single-dose container
Discard unused portion.
NOT FOR INTRATHECAL USE
Dose: See package insert. For intravascular use only. Each mL contains 498.72 mg iopromide with 2.42 mg tromethamine as a buffer and 0.1 mg edetate calcium disodium as a stabilizer.
Contains no antimicrobial preservative.
Protect from light.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Mfd. for:
Bayer HealthCare Pharmaceuticals Inc.
Wayne, NJ 07470
Mfd in Germany
50 mL
240
Package/Label Display Panel 300 mg Iodine per 50mL
NDC 50419-344-05 50 mL
sterile solution
Ultravist®
(brand of iopromide)
300 mgI/mL injection
Rx only
Single-dose container
Discard unused portion.
NOT FOR INTRATHECAL USE
Dose: See package insert. For intravascular use only. Each mL contains 623.4 mg iopromide with 2.42 mg tromethamine as a buffer and 0.1 mg edetate calcium disodium as a stabilizer.
Contains no antimicrobial preservative.
Protect from light.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Mfd. for:
Bayer HealthCare Pharmaceuticals Inc.
Wayne, NJ 07470
Mfd in Germany
50 mL
300
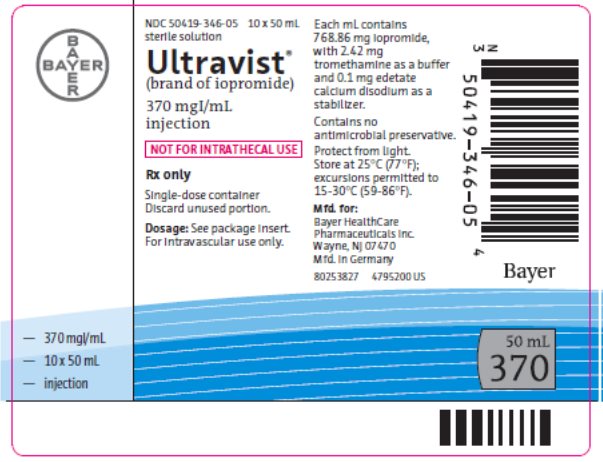
Package/Label Display Panel 370 mg Iodine per 50mL
NDC 50419-346-05 50 mL
sterile solution
Ultravist®
(brand of iopromide)
370 mgI/mL injection
Rx only
Single-dose container
Discard unused portion.
NOT FOR INTRATHECAL USE
Dose: See package insert. For intravascular use only. Each mL contains 768.86 mg iopromide with 2.42 mg tromethamine as a buffer and 0.1 mg edetate calcium disodium as a stabilizer.
Contains no antimicrobial preservative.
Protect from light.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Mfd. for:
Bayer HealthCare Pharmaceuticals Inc.
Wayne, NJ 07470
Mfd in Germany
Bayer
50 mL
370
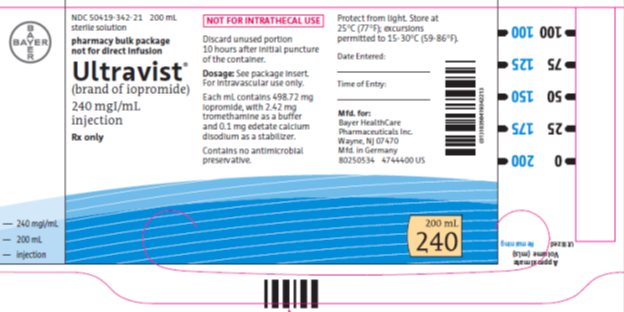
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 50419-342-21 200 mL
sterile solution
pharmacy bulk package
not for direct infusion
Ultravist®
(brand of iopromide)s
240 mg Iodine per mL
injection
Rx only
NOT FOR INTRATHECAL USE
Discard unused portion 10 hours after initial puncture of the container.
Dose: See package insert. For intravascular use only.
Each mL contains 498.72 mg iopromide with 2.42 mg tromethamine as a buffer and 0.1 mg edetate calcium disodium as a stabilizer.
Contains no antimicrobial preservative.
Protect from light.
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Mfd. for:
Bayer HealthCare Pharmaceuticals Inc.
Wayne, NJ 07470
Mfd in Germany
200 mL
240