AXE FREEZE ANTIDANDRUFF- pyrithione zinc shampoo
Conopco Inc. d/b/a Unilever
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
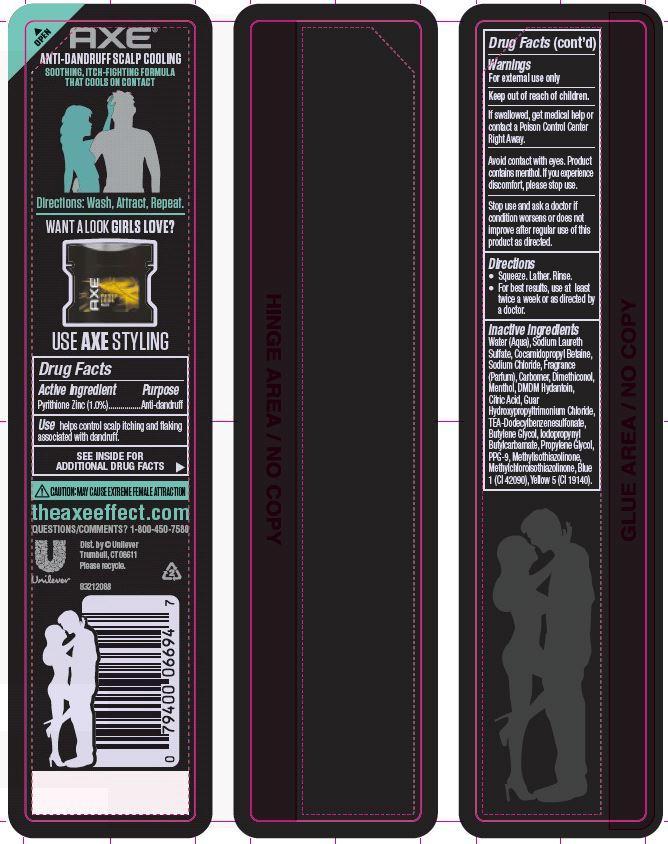
Active Ingredient
Pyrithione Zinc (1.0%)
Use helps control scalp itching and flaking associated with dandruff.
Warnings
For external use only
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center Right Away.
Avoid contact with eyes. Product contains menthol. If you experience discomfort, please stop use.
Stop use and ask a doctor if condition worsens or does not improve after regular use of this product as directed.
Direction
· Squeeze. Lather. Rinse
· For best results, use at least twice a week or as directed by a doctor.
Inactive ingredients
Water (Aqua), Sodium Laureth Sulfate, Cocamidopropyl Betaine, Sodium Chloride, Fragrance (Parfum), Carbomer, Dimethiconol, Menthol, DMDM Hydantoin, Citric Acid, Guar Hydroxypropyltrimonium Chloride, TEA-Dodecylbenzenesulfonate, Butylene Glycol, Iodopropynyl Butylcarbamate, Propylene Glycol, PPG-9, Methylisothiazolinone, Methylchloroisothiazolinone, Blue 1 (CI 42090), Yellow 5 (CI 19140).
theaxeeffect.com
QUESTIONS/COMMENTS?
1-800-450-7580
12 FL OZ PDP

12 FL OZ back
Conopco Inc. d/b/a Unilever

