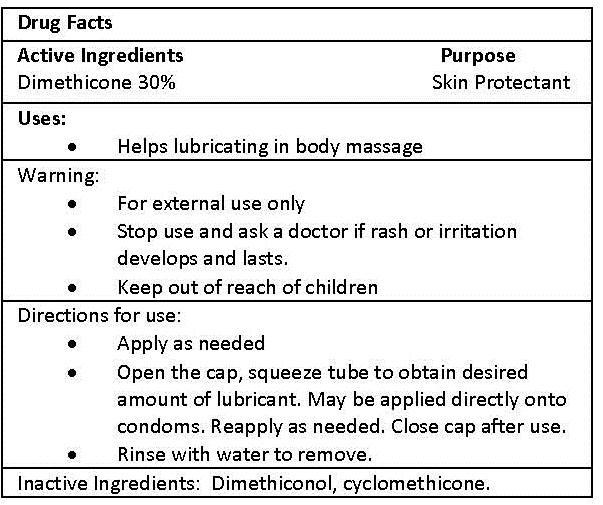
EROS ANAL SILICONE- dimethicone gel
MEGASOL COSMETIC GMBH
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| EROS ANAL SILICONE
dimethicone gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - MEGASOL COSMETIC GMBH (344152855) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MEGASOL COSMETIC GMBH | 344152855 | manufacture(53389-410) | |
Revised: 1/2018
Document Id: 3c4f84f7-ba53-424b-930f-c68ed5ae1ae0
Set id: de7dca2d-f7fd-4723-a314-0c08c1bf47d8
Version: 3
Effective Time: 20180116
MEGASOL COSMETIC GMBH