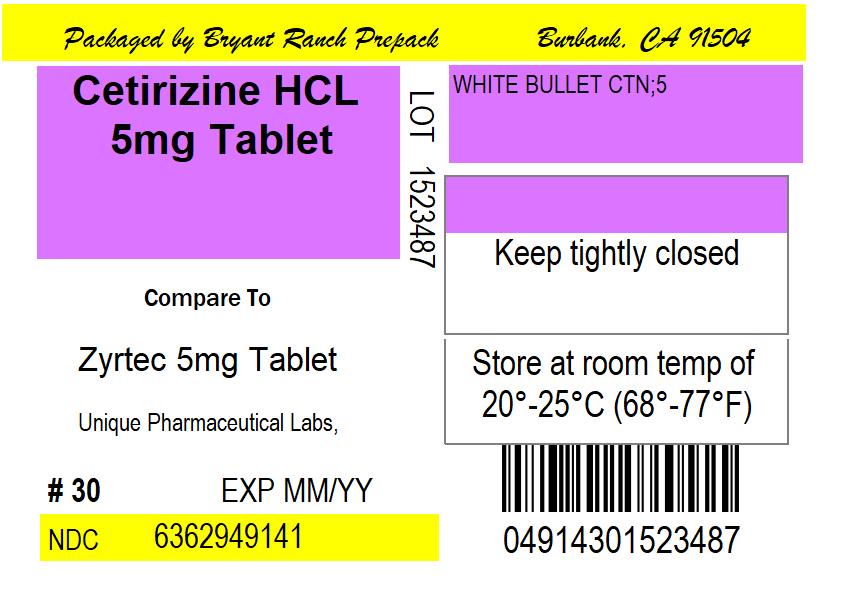
USES
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
WARNINGS:
DO NOT USE
Do Not Use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
ASK DOCTOR
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
ASK DOCTOR/PHARMACIST
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
WHEN USING THIS PRODUCT
- drowsines may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinary.
STOP USE
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
DIRECTIONS
|
Adults and children 6years and over |
1 to 2 tablets once daily depending upon severity of symptoms; do not take more than 2 tablets in 24 hours |
|
Adults 65 years and over |
1 tablet once a day; do not take more than 1 tablet in 24 hours |
|
Children under 6 years of age |
Ask a doctor |
|
Consumers with liver or kidney disease |
Ask a doctor |
INACTIVE INGREDIENTS
hypromellose, lactose, magnesium stearate, maize starch, polyethylene glycol, povidone, titanium dioxide.