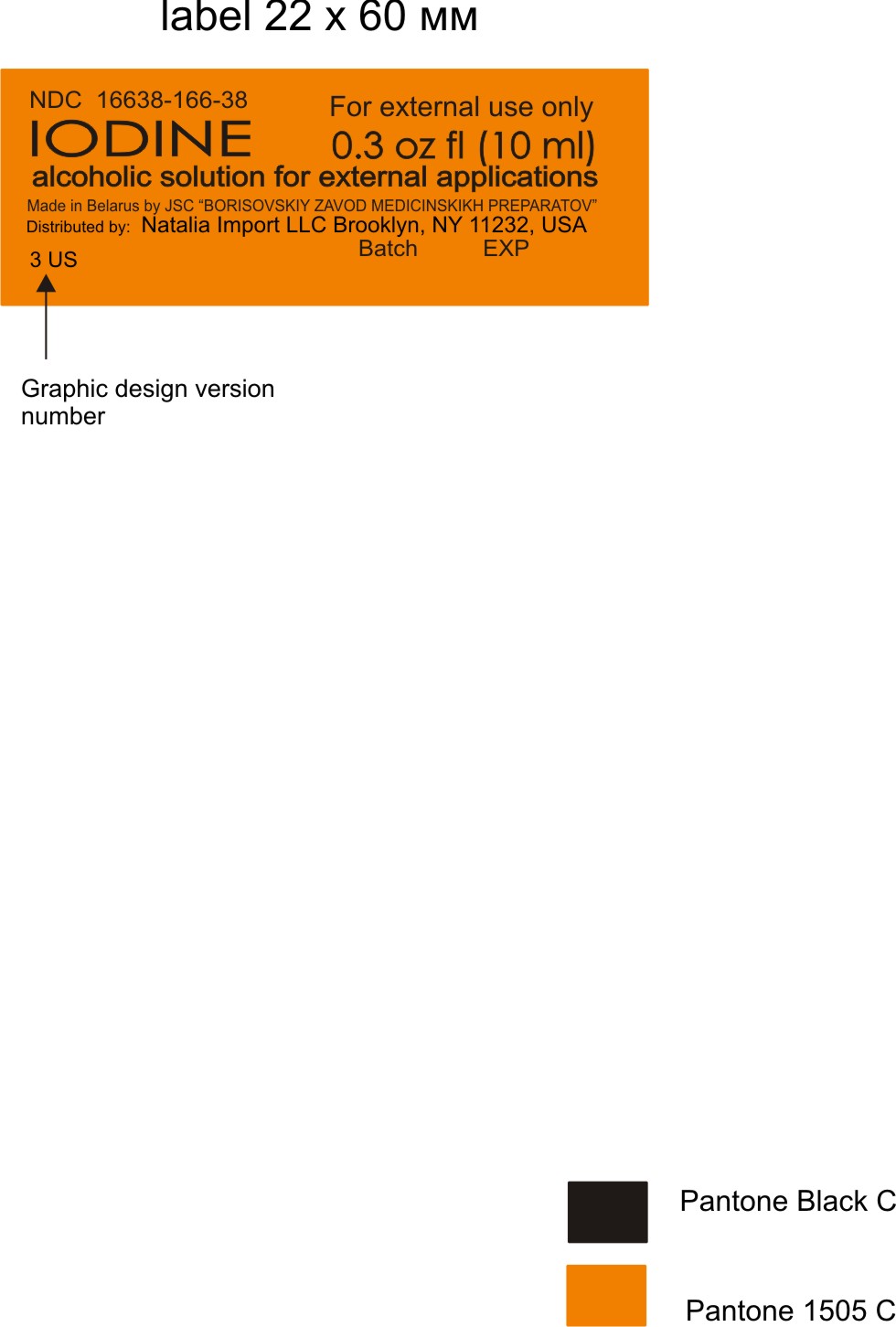
IODINE- iodine alcoholic solution
Borisovskiy Zavod Medicinskikh Preparatov JSC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Iodine - 0.5 g, Potassium Iodide - 0.2 g
Purpose
Bactericidial - antiseptic
Uses
For inflammatory and infectious diseases of skin, cuts, minor injuries, wounds, myositis, neuraldia; for disinfection of the operational field.
Warnings
For external use only.
Avoid contact with eyes. If contact occurs, rinse eyes throughly with water.
Stop use and ask a doctor if condition worsens or does not improve after regular use of this product as directed.
Keep out of reach of children. If swallowed, seek medical attention or contact a Poison Control center immediately.
Directions
Apply to affected areas as needed or as directed by physician.
Other information
Keep in a dark place at temperature below 25
oC.
Inactive ingredients
Ethanol, Purified Water