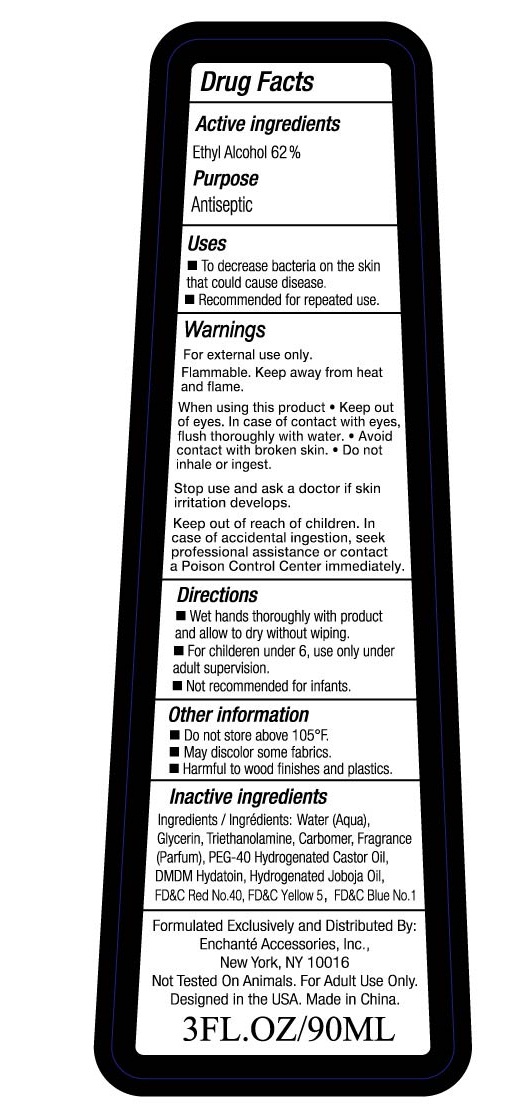
Warnings:
For external use only
Flammable. Keep away from heat and flame.
When using this product
- Keep out of eyes. In case of contact with eyes, flush thoroughly with water
- Avoid contact with broken skin
- Do not inhale or ingest
Stop use and ask a doctor if skin irritation develops
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions:
- Wet hands thoroughly with product and allow to dry without wiping
- For children under 6, use only under adult supervision.
- Not recommended for infants
Other Information
- Do not store above 105F
- May discolor some fabrics
- Harmful to wood finishes and plastics