FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
NitroMist is indicated for acute relief of an attack or acute prophylaxis of angina pectoris due to coronary artery disease.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
At the onset of an attack, one metered spray or two metered sprays should be administered on or under the tongue. A spray may be repeated approximately every 5 minutes as needed. If two sprays are used initially, the patient may only administer one more spray after waiting 5 minutes. No more than 3 metered sprays are recommended within a 15-minute period. If chest pain persists after a total of 3 sprays, prompt medical attention is recommended. NitroMist may be used prophylactically 5 minutes to 10 minutes before engaging in activities that might precipitate an acute attack.
2.2 Priming the Container
After an initial priming of 10 sprays, each metered spray of NitroMist delivers 33 mg of solution containing 400 mcg of nitroglycerin. It will remain adequately primed for 6 weeks. If the product is not used within 6 weeks, it can be adequately re-primed with 2 sprays. NitroMist is available in either 230 metered sprays or 90 metered sprays per container, but the total number of available doses depends on the number of sprays per use (1 spray or 2 sprays), and the frequency of priming.
2.3 Administration
During use the patient should rest, ideally in the sitting position. The container should be held vertically with the valve head uppermost and the spray orifice as close to the mouth as possible. The dose should preferably be sprayed into the mouth on or under the tongue by pressing the button firmly and the mouth should be closed immediately after each dose. THE SPRAY SHOULD NOT BE INHALED. Patients should be instructed to familiarize themselves with the position of the spray orifice, which can be identified by the finger rest on top of the valve, in order to facilitate orientation for administration at night.
- Do not shake container.
- Remove plastic cap.
- If this is the first time using the bottle, press actuator button 10 times to ensure proper dose priming (holding unit away from yourself and others).
- Hold container upright with forefinger on top of the actuator button.
- Open mouth and bring the container as close as possible.
- Press the actuator button firmly with forefinger to release spray(s) onto or under the tongue.
- Release button and close mouth. The medication should not be spit out or the mouth rinsed for 5 minutes to 10 minutes following administration.
- If a second administration is required to obtain relief, repeat steps 4, 5, and 6. No more than 3 metered sprays can be given within a 15-minute period.
- Replace plastic cover.
- If the product is not used for more than 6 weeks, then it can be adequately re-primed with 2 sprays.
The level of the liquid in the container should be periodically checked. While the container is in the upright position, if the liquid reaches the top or middle of the hole on the side of the container, one should order more. When the liquid reaches the bottom of the hole, the remaining doses will have less than label content.
3 DOSAGE FORMS AND STRENGTHS
Lingual aerosol, 400 mcg per spray, is available in either 230 metered sprays or 90 metered sprays per container.
4 CONTRAINDICATIONS
4.1 PDE5 Inhibitor Use
Administration of NitroMist is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), as PDE5 inhibitors such as sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates. Do not use NitroMist in patients who are taking the soluble guanylate cyclase (sGC) stimulator riociguat. Concomitant use can cause hypotension. [see DRUG INTERACTIONS (7.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Tolerance
Excessive use may lead to the development of tolerance. Only the smallest number of doses required for effective relief of the acute anginal attack should be used [see DOSAGE AND ADMINISTRATION (2)].
As tolerance to other forms of nitroglycerin develops, the effect of sublingual nitroglycerin on exercise tolerance, although still observable, is reduced.
5.2 Hypotension
Severe hypotension, particularly with upright posture, may occur even with small doses of nitroglycerin. The drug should therefore be used with caution in patients who may be volume-depleted or who, for whatever reason, are already hypotensive. Hypotension induced by nitroglycerin may be accompanied by paradoxical bradycardia and increased angina pectoris.
The benefits of NitroMist in patients with acute myocardial infarction or congestive heart failure have not been established. If one elects to use NitroMist in these conditions, careful clinical or hemodynamic monitoring must be used because of the possibility of hypotension and tachycardia.
6 ADVERSE REACTIONS
6.1 Headache
Headache, which may be severe and persistent, may occur immediately after nitroglycerin use.
6.2 Flushing
Flushing, drug rash and exfoliative dermatitis have been reported in patients receiving nitrate therapy.
6.3 Hypotension
Postural hypotension, as manifest by vertigo, weakness, palpitation, and other symptoms, may develop occasionally, particularly in erect, immobile patients. Marked sensitivity to the hypotensive effects of nitrates (manifested by nausea, vomiting, weakness, diaphoresis, pallor, and collapse) may occur at therapeutic doses.
7 DRUG INTERACTIONS
7.1 PDE5 Inhibitors
Administration of NitroMist is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). PDE5 inhibitors such as sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates. Do not use NitroMist in patients who are taking the soluble guanylate cyclase (sGC) stimulator riociguat. Concomitant use may result in severe hypotension, syncope, or myocardial ischemia.
The time course and dose dependence of this interaction have not been studied, and use within a few days of one another cannot be recommended. Appropriate supportive care for the severe hypotension has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion. The use of any form of nitroglycerin during the early days of acute myocardial infarction requires particular attention to hemodynamic monitoring and clinical status.
7.2 Antihypertensives
Patients receiving antihypertensive drugs, beta-adrenergic blockers, and nitrates should be observed for possible additive hypotensive effects. Marked orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used concomitantly.
Labetolol blunts the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effects. If labetolol is used with nitroglycerin in patients with angina pectoris, additional hypotensive effects may occur.
7.3 Aspirin
Coadministration of aspirin and nitroglycerin has been reported to result in increased nitroglycerin maximum concentrations by as much as 67% and AUC by 73% when administered as a single dose. The vasodilatory and hemodynamic effects of nitroglycerin may be enhanced by concomitant administration of aspirin.
7.4 Tissue-type Plasminogen Activator (t-PA)
Intravenous administration of nitroglycerin decreases the thrombolytic effect of tissue-type plasminogen activator (t-PA). Plasma levels of t-PA are reduced when coadministered with nitroglycerin. Therefore, caution should be observed in patients receiving nitroglycerin during t-PA therapy.
7.5 Heparin
Intravenous nitroglycerin reduces the anticoagulant effect of heparin. Activated partial thromboplastin times (APTT) should be monitored in patients receiving heparin and intravenous nitroglycerin. It is not known if this effect occurs following single nitroglycerin doses.
7.6 Ergotamine
Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and subsequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore, patients receiving sublingual nitroglycerin should avoid ergotamine and related drugs or be monitored for symptoms of ergotism if this is not possible.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published data on the use of nitroglycerin are insufficient to determine a drug associated risk of major birth defects or miscarriage. In animal reproduction studies, there were no adverse developmental effects when nitroglycerin was administered intravenously to rabbits or intraperitoneally to rats during organogenesis at doses greater than 64-times the human dose [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 – 4% and 15 – 20%, respectively.
Data
Animal Data
No embryotoxic or postnatal development effects were observed with transdermal application in pregnant rabbits and rats at doses up to 240 mg/kg/day for 13 days, at intraperitoneal doses in pregnant rats up to 20 mg/kg/day for 11 days, and at intravenous doses in pregnant rabbits up to 4 mg/kg/day for 13 days.
8.2 Lactation
Risk Summary
Sublingual nitroglycerin has not been studied in lactating women. It is not known if nitroglycerin is present in human milk, or if nitroglycerin has effects on milk production or the breastfed child. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s need for nitroglycerin and any potential adverse effects on the breastfed child from nitroglycerin or from the underlying maternal condition.
8.3 Pediatric Use
The safety and effectiveness of nitroglycerin in pediatric patients have not been established.
8.4 Geriatric Use
Clinical studies of NitroMist did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly (greater than or equal to 65 years) and younger (less than 65 years) patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
Signs and symptoms of hemodynamic effects: The effects of nitroglycerin overdose are generally the results of nitroglycerin’s capacity to induce vasodilatation, venous pooling, reduced cardiac output, and hypotension. These hemodynamic changes may have protean manifestations, including increased intracranial pressure with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo; palpitations; tachycardia; visual disturbances; nausea and vomiting (possibly with colic and even bloody diarrhea); syncope (especially in the upright posture); dyspnea, later followed by reduced ventilatory effort, diaphoresis, with the skin either flushed or cold and clammy; heart block and bradycardia; paralysis; coma; seizures; and death.
No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been subject to controlled study as a therapy of nitroglycerin overdose. Because the hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient’s legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary.
The use of epinephrine or other arterial vasoconstrictors in this setting is not recommended.
In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of nitroglycerin overdose in these patients may be subtle and difficult, and invasive monitoring may be required.
Methemoglobinemia: Methemoglobinemia has been rarely reported with organic nitrates. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate arterial PO 2. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air.
If methemoglobinemia is present, intravenous administration of methylene blue, 1 mg/kg to 2 mg/kg of body weight, may be required.
11 DESCRIPTION
Nitroglycerin, an organic nitrate, is a vasodilator which has effects on both arteries and veins. The chemical name for nitroglycerin is 1,2,3-propanetriol trinitrate (C 3H 5N 3O 9). The compound has a molecular weight of 227.09. The chemical structure is:
CH 2 – ONO 2
|
CH – ONO 2
|
CH 2 – ONO 2
NitroMist (nitroglycerin) lingual aerosol is a metered-dose spray containing 230 metered sprays or 90 metered sprays of nitroglycerin per container. This product delivers 400 mcg of nitroglycerin per actuation in the form of spray droplets on or under the tongue. Inactive ingredients: caprylic/capric diglycerol succinate, peppermint oil, L(-)-menthol, n-butane.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nitroglycerin forms free radical nitric oxide (NO), which activates guanylate cyclase, resulting in an increase of guanosine 3’,5’-monophosphate (cyclic GMP) in smooth muscle and other tissues. This eventually leads to dephosphorylation of myosin light chains, which regulates the contractile state in smooth muscle and results in vasodilatation.
12.2 Pharmacodynamics
The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle. Although venous effects predominate, nitroglycerin produces, in a dose-related manner, dilation of both arterial and venous beds. Dilation of the postcapillary vessels, including large veins, promotes peripheral pooling of blood, decreases venous return to the heart, and reduces left ventricular end-diastolic pressure (preload). Nitroglycerin also produces arteriolar relaxation, thereby reducing peripheral vascular resistance and arterial pressure (after load), and dilates large epicardial coronary arteries; however, the extent to which this latter effect contributes to the relief of exertional angina is unclear.
Therapeutic doses of nitroglycerin may reduce systolic, diastolic and mean arterial blood pressure. Effective coronary perfusion pressure is usually maintained, but can be compromised if blood pressure falls excessively or increased heart rate decreases diastolic filling time.
Elevated central venous and pulmonary capillary wedge pressures, and pulmonary and systemic vascular resistance are also reduced by nitroglycerin therapy. Heart rate is usually slightly increased, presumably a reflex response to the fall in blood pressure. Cardiac index may be increased, decreased, or unchanged. Myocardial oxygen consumption or demand (as measured by the pressure-rate product, tension-time index, and stroke-work index) is decreased and a more favorable supply-demand ratio can be achieved. Patients with elevated left ventricular filling pressure and increased systemic vascular resistance in association with a depressed cardiac index are likely to experience an improvement in cardiac index. In contrast, when filling pressures and cardiac index are normal, cardiac index may be slightly reduced following nitroglycerin administration.
12.3 Pharmacokinetics
Nitroglycerin is rapidly absorbed following lingual spray administration. In a pharmacokinetic study when a single 1200 mcg dose (three activations of a 400 mcg dose) of NitroMist was administered to healthy volunteers (n=12), all subjects had detectable trinitroglycerin plasma levels (mean C max 0.8 ng/mL ± 0.7 ng/mL and t max of 8 minutes, range 4 minutes to 15 minutes) beginning at 2 minutes post-dose and higher levels of the 1,2- (mean C max 3.7 ng/mL ± 1 ng/mL and t max 34 minutes ± 21 minutes, range 15 minutes to 90 minutes) and 1,3-dinitroglycerin metabolites (mean C max 1 ng/mL ± 0.3 ng/mL and mean t max 41 minutes ± 20 minutes, range 20 minutes to 90 minutes).
The volume of distribution of nitroglycerin following intravenous administration is 3.3 L/kg.
A liver reductase enzyme is of primary importance in the metabolism of nitroglycerin to glycerol di- and mononitrate metabolites and ultimately to glycerol and organic nitrate. Known sites of extrahepatic metabolism include red blood cells and vascular walls. In addition to nitroglycerin, 2 major metabolites, 1,2- and 1,3-dinitroglycerin are found in plasma. The mean elimination half-life of both 1,2- and 1,3-dinitroglycerin is about 40 minutes. The 1,2- and 1,3-dinitroglycerin metabolites have been reported to possess some pharmacological activity, whereas the glycerol mononitrate metabolites of nitroglycerin are essentially inactive. Higher plasma concentrations of the dinitro metabolites, with their nearly 8-fold longer elimination half-lives, may contribute significantly to the duration of pharmacologic effect.
In the above referenced pharmacokinetic study the average initial half-lives (T ½α) of nitroglycerin, and its 1,2- and 1,3-dinitroglycerin metabolites were estimated to be 3 minutes, 10 minutes, and 11 minutes, respectively. The half-life of disappearance of the nitroglycerin (T ½β) (5 minutes) was significantly less than the half-life of appearance (T ½α) of the 1,2- and 1,3-dinitroglycerin metabolites suggesting the possibility of an additional compartment into which the nitroglycerin disappears from plasma prior to being metabolized into the dinitroglycerin metabolites. A second indication of this other compartment is that the appearance of nitroglycerin metabolites in plasma was delayed in some subjects, with zero plasma levels seen for 4 minutes to 6 minutes after dosing. In some subjects, nitroglycerin metabolites appeared only after nitroglycerin C max had been observed.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal carcinogenicity studies with sublingually administered or lingual spray nitroglycerin have not been performed.
Rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years developed dose-related fibrotic and neoplastic changes in liver, including carcinomas, and interstitial cell tumors in testes. At the highest dose, the incidences of hepatocellular carcinomas was 52% compared to 0% in untreated controls. Incidences of testicular tumors were 52% vs 8% in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice.
Nitroglycerin was found to have reverse mutation activity in the Salmonella typhimurium strain TA1535 (Ames assay). A similar mutation in S. typhimurium strain was also reported for other NO donors. Nevertheless, there was no evidence of mutagenicity in an in vivo dominant lethal assay with male rats treated with oral doses of up to about 363 mg/kg/day or in ex vitro cytogenic tests in rat and dog tissues. In vitro cytogenetic assay using Chinese hamster ovary cells showed no chromosomal aberrations.
In a 3-generation reproduction study, rats received dietary nitroglycerin at doses up to about 408 mg/kg/day (males) to 452 mg/kg/day (females) for 5 months (females) or 6 months (males) prior to mating of the F 0 generation with treatment continuing through successive F 1 and F 2 generations. The highest dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F 0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males.
14 CLINICAL STUDIES
In a randomized, double-blind, single-center, single-administration, placebo-controlled, 4-period cross-over study in 30 subjects with stable angina pectoris, statistically significant dose-related increases in exercise tolerance were seen following doses of 200 mcg, 400 mcg, and 800 mcg of nitroglycerin delivered by NitroMist compared to placebo.
16 HOW SUPPLIED/STORAGE AND HANDLING
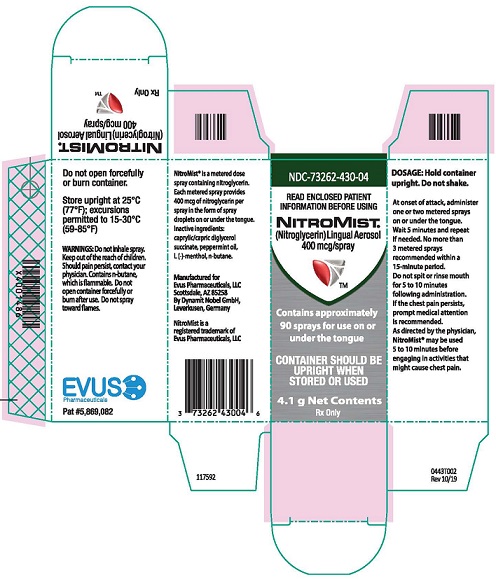
Each box of NitroMist contains one glass bottle coated with red/orange transparent plastic which assists in containing the glass and medication should the bottle be shattered. NitroMist is available as an 8.5 g (Net Content) of nitroglycerin lingual aerosol that will deliver 230 metered sprays containing 400 mcg of nitroglycerin per actuation or as a 4.1 g (Net Content) of nitroglycerin lingual aerosol that will deliver 90 metered sprays containing 400 mcg of nitroglycerin per actuation.
230 metered sprays: NDC 73262-430-08
90 metered sprays: NDC 73262-430-04
- Storage
Store at room temperature (25 °C, 77 °F); excursions permitted to 15 ° to 30 °C (59 ° to 85 °F)
- Handling
NitroMist contains a highly flammable propellant (butane). Do not forcefully open a NitroMist bottle, do not have the container burned after use, and do not spray directly toward flames.
17 PATIENT COUNSELING INFORMATION
17.1 Interaction with PDE5 Inhibitors
NitroMist should not be used in patients who are using medications for erectile dysfunction such as sildenafil, vardenafil, and tadalafil. These products have been shown to increase the hypotensive effects of nitrate drugs such as NitroMist.
17.2 Administration
Patients should be instructed that prior to initial use of NitroMist Lingual aerosol, the pump must be primed by pressing the actuator button 10 times to ensure proper dose priming. If the product is not used for more than 6 weeks, the bottle can be adequately re-primed with 2 sprays.
NitroMist is meant to be sprayed on or under the tongue at the beginning of angina or to prevent an angina attack. Treatment with nitroglycerin products such as NitroMist may be associated with lightheadedness on standing, especially just after rising from a laying or seated position. This effect may be more frequent in patients who have consumed alcohol, since alcohol use contributes to hypotension. If possible, patients should be seated when taking NitroMist. This reduces the likelihood of falling due to lightheadedness or dizziness [ see DOSAGE AND ADMINISTRATION (2.3)].
17.3 Headache
Headaches can sometimes accompany treatment with nitroglycerin. In patients who get these headaches, the headaches may indicate activity of the drug. Tolerance to headaches develops.
17.4 Flushing
Flushing, drug rash and exfoliative dermatitis have been reported in patients receiving nitrate therapy.
17.5 Container information
The NitroMist bottle should not be forcefully opened. Because NitroMist contains a highly flammable propellant (butane), do not have the container burned after use and do not spray directly towards flames.
While the container is in the upright position, if the liquid reaches the top to middle of the hole on the side of the container, a new supply should be obtained. When the liquid reaches the bottom of the hole, the remaining doses will have less than label content .
Manufactured for Evus Pharmaceuticals, LLC

Scottsdale, AZ 85258 USA
by Dynamit Nobel GmbH, Leverkusen, Germany
Marketed and Distributed by: Evus Pharmaceuticals, LLC
Scottsdale, AZ 85258 USA
NitroMist is a registered trademark of Evus Pharmaceuticals
141110
143F003
Revised 09/2019
PRINCIPAL DISPLAY PANEL
NDC 73262-430-08
NITROMIST
(Nitroglycerin) Lingual Aerosol
400 mcg/spray
contains approximately
230 sprays for use on or
under the tongue
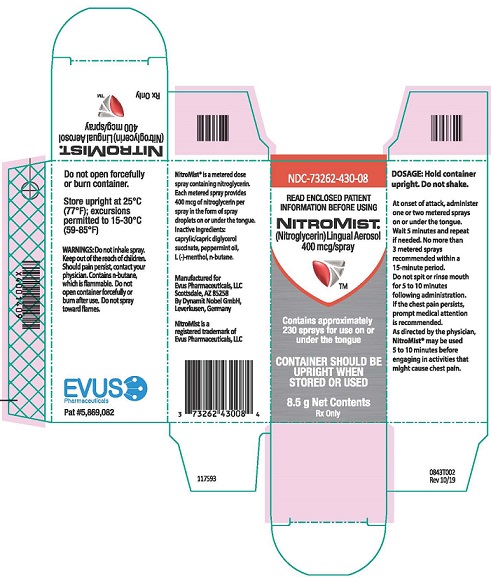
PRINCIPAL DISPLAY PANEL
NDC 73262-430-08
NITROMIST
(Nitroglycerin) Lingual Aerosol
400 mcg/spray
contains approximately
230 sprays for use on or
under the tongue