Do not use
on wounds.
on irritated or damaged skin.
if the appearance of this product has changed.
if you are hypersensitive to this product.
otherwise than as directed.
When using this product
avoid contact with the eye or mucous membranes.
supervise use by children.
use caution if prone to allergic reactions.
Stop Use and Ask a Doctor If
condition worsens.
symptoms persist for more than 7 days.
symptoms clear up and occur again within a few days.
excessive irritation of the skin develops.
redness is present.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
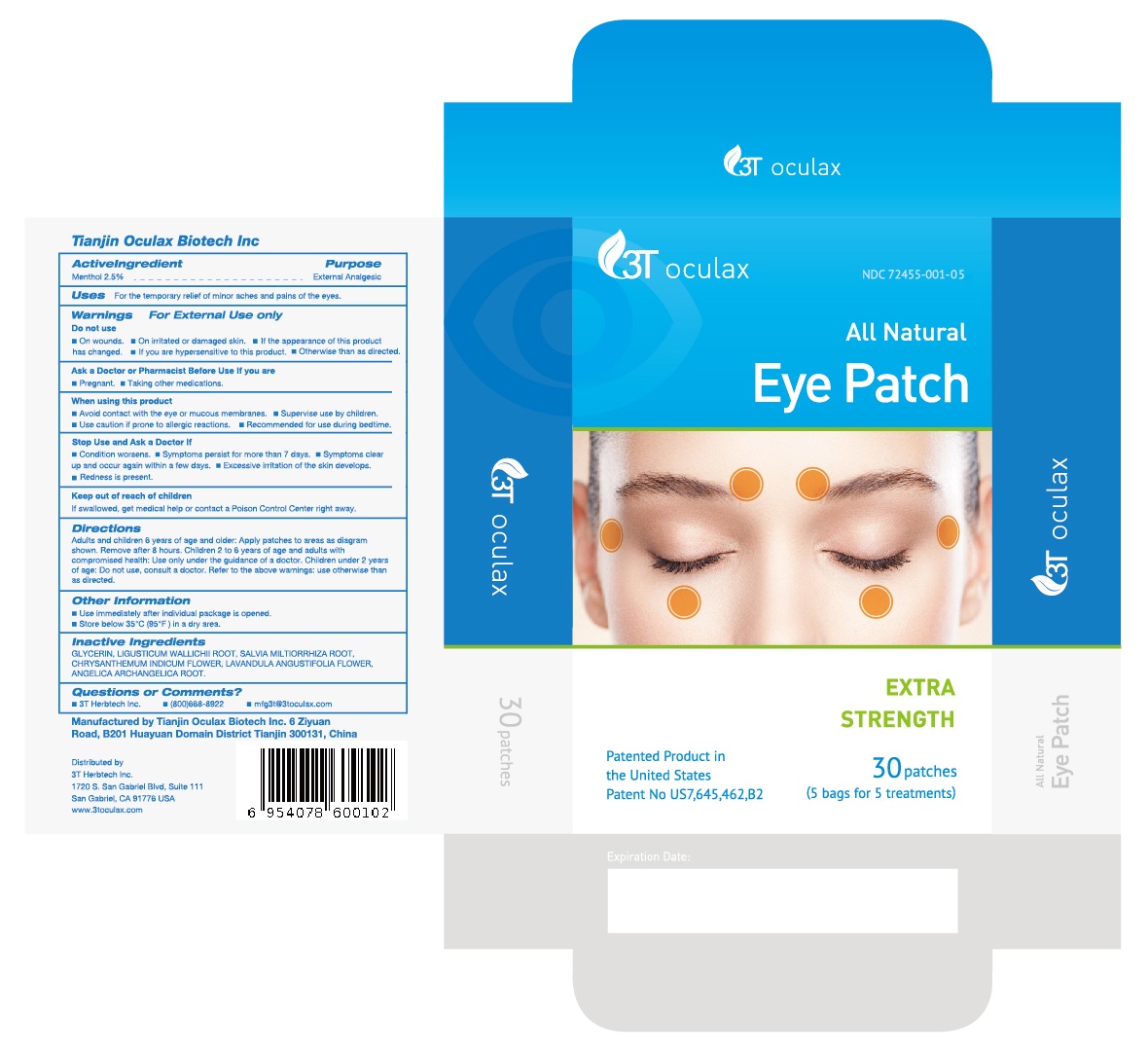
Directions
Adults and children 6 years of age and older: Apply patches to areas as diagram shown. Remove after 8 hours. Children 2 to 6 years of age and adults with compromised health: Use only under the guidance of a doctor. Children under 2 years of age: Do not use, consult a doctor. Refer to the above warnings: use otherwise than as directed.
Other Information
Use immediately after individual package is opened.
Store below 35 ℃ (95 ℉) in a dry area.
Inactive Ingredients
GLYCERIN, LIGUSTICUM WALLICHII ROOT, SALVIA MILTIORRHIZA ROOT, CHRYSANTHEMUM INDICUM FLOWER, LAVANDULA ANGUSTIFOLIA FLOWER, ANGELICA ARCHANGELICA ROOT