What is a Migraine?
Migraines are classified as “benign headaches” because they are not caused by an underlying medical condition such as a tumor, aneurysm or a similar medical disorder. But, migraine is far from “benign” in terms of its impact on the individual and those around them.
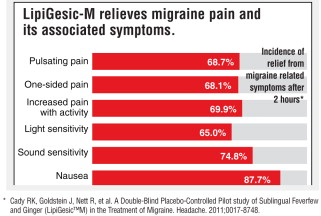
The pain of a migraine headache is often described as an intense pulsing, throbbing and sometimes disabling pain, which may be limited to one side of the head. It may be accompanied by sensitivity to light and sound, nausea and vomiting.
There are two major types of migraine headache: classic and common. Classic migraines are often signaled by “aura,” a visual sensory illusion, such as jagged bands of light obscuring vision, or shimmering lights around the edges of objects, before the actual pain begins. Only about 20 percent of sufferers experience aura. Common migraines occur without aura.
Migraine headaches may result from dietary, environmental and lifestyle triggers. Most migraines are not caused by a single identifiable source, and a person's response to a trigger varies from occurrence to occurrence.
Migraine Triggers
DIETARY TRIGGERS
- Alcoholic drinks (esp. red wine) Changes in caffeine intake
- Aged cheese
- Chocolate
- Monosodium glutamate (MSG) Nitrates (food preservatives) Yeast
- Smoked fish
- Nuts
- Pickled foods
- Missed meals
What will LipiGesic™-M do for me?
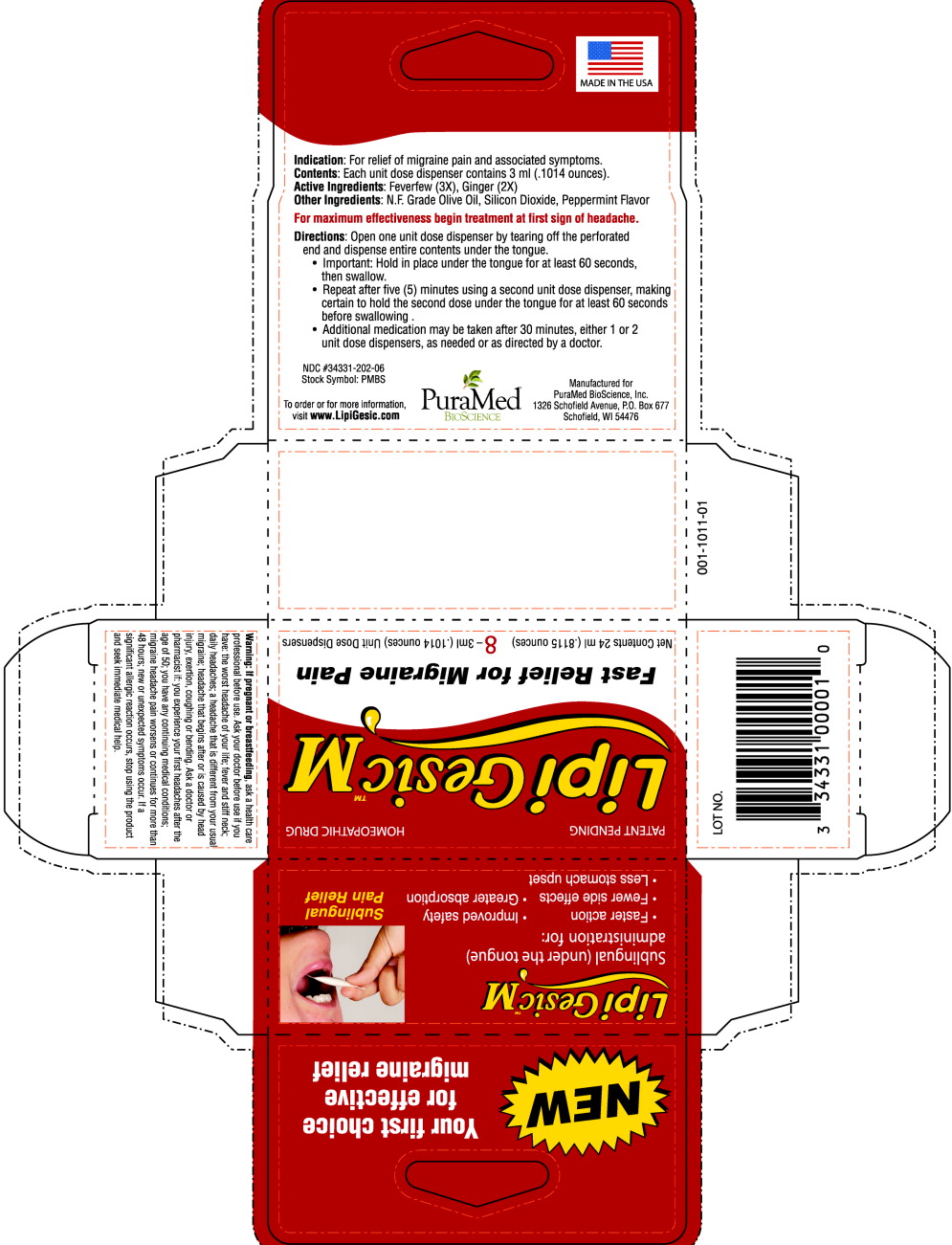
LipiGesic-M is a non-prescription sublingual (under-the-tongue) formulation of natural ingredients used in the early stages of onset to relieve the pain and reduce the associated symptoms of migraine – nausea, vomiting, and sensitivity to light and sound.
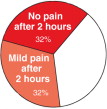
In a double-blind placebo controlled study, 64% of headaches resolved with no pain or only mild pain, within 2 hours after LipiGesic-M was taken. (In most cases, relief was experienced prior to the 2-hour- study benchmark.)
LipiGesic-M is highly effective for the treatment of migraine pain and may cost up to 75% less than the leading prescription migraine medications. Available over-the-counter, LipiGesic-M is the best, first-line defense in the treatment of migraine. Each dose of LipiGesic-M is pre-measured; it is easy to use and easy to transport.
WARNING:
If pregnant or breast- feeding, ask a health care professional before use.
Also, you should talk to you doctor before using if you have: the worst headache of your life; fever and stiff neck; daily headaches; a headache that is different from your usual migraine; headache that begins after or is caused by head injury, exertion, coughing or bending; experience your first headaches after the age of 50; any continuing medical conditions; migraine headache pain that worsens or continues for more than 48 hours; new or unexpected symptoms.
If a significant allergic reaction occurs, stop using the product and seek immediate medical help.
DIRECTIONS
For maximum effectiveness, begin treatment at first sign of headache.
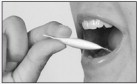
- Open one unit dose dispenser by tearing off the perforated end and dispensing entire contents under the tongue.
Important: Hold in place under the tongue for at least 60 seconds; then swallow.
- Repeat after 5 minutes using a second unit dose dispenser, hold the second dose under the tongue for at least 60 seconds before swallowing.
- Additional medication may be taken after 30 minutes, either I or 2 unit dose dispensers, as needed or as directed by doctor.
LipiGesic™-M is fast.
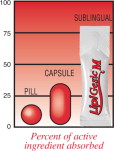
Medications absorbed through the digestive tract generally take up to an hour just to start working, and absorption is highly variable. With the sublingual unit dose system, LipiGesic-M achieves consistent absorption that begins within minutes.
LipiGesic-M is powerful.
LipiGesic-M's patent-pending formulation, has been shown to be a safe and effective first-line treatment when used at the onset of migraine.* LipiGesic-M is non-habit forming and not associated with rebound headache.
The active ingredients, Feverfew and Ginger, have had a long history of use and have an excellent safety profile. They have been combined to provide a unique and powerful defense against migraine pain and associated symptoms.
It's all in the delivery.
LipiGesic™-M is a patent - pending medication designed to provide fast and effective relief from the pain and associated symptoms of migraine. Unlike typical pills and capsules, LipiGesic-M starts to work almost immediately
Active ingredients are often destroyed when first passing through the stomach or liver – before they have a chance to reach their intended target. Sublingual (under the tongue) administration prevents this “first pass” destruction by delivering the product directly to the bloodstream through the rich network of blood vessels under the tongue. LipiGesic-M's full- strength active ingredients reach the bloodstream faster and more effectively.
The unit dose system is a patent-pending delivery system developed by PuraMed BioScience, and includes the dispenser as well as specific formula enhancements used to improve the sublingual absorption of LipiGesic-M. The unit dose system is an important part of PuraMed's effort to create products that work faster, are more effective and offer better safety profiles than similar products administered by traditional means.
PuraMed™
BIOSCIENCE
Manufactured for
PuraMed BioScience, Inc. P.O. Box 677, 1326 Schofield Ave. Schofield, WI 54476
For more information:
www.LipiGesic.com
877-851-2190
info@LipiGesic.com
© PuraMed BioScience 2011 002-1011-1