CAUTION: Federal law restricts medicated feed containing
this veterinary feed directive (VFD) drug to use by or on
the order of a licensed veterinarian.
For Use in Swine and Beef Cattle Feeds Only
Do Not Feed Undiluted
Equivalent to 100 g Tylosin per Pound
Swine:
For the reduction in severity of effects of atrophic rhinitis.
For control of swine dysentery associated with Brachyspira hyodysenteriae.
For the treatment and control of swine dysentery associated with Brachyspira
hyodysenteriae immediately after medicating with Tylovet Soluble (tylosin)
drinking water.
For control of porcine proliferative enteropathies (PPE, ileitis) associated with
Lawsonia intracellularis.
For control of porcine proliferative enteropathies (PPE, ileitis) associated with
Lawsonia intracellularis immediately after medicating with Tylovet Soluble (tylosin)
in drinking water.
Beef Cattle:
For reduction of incidence of liver abscesses associated with Fusobacterium
necrophorum and Arcanobacterium pyogenes.
Restricted Drug (California) Use Only as Directed
Approved by FDA under ANADA # 200-484
Huvepharma and Tylovet are trademarks of Huvepharma EOOD.
Distributed By:
Huvepharma, Inc
525 Westpark Drive, Suite 230
Peachtree City, GA 30269
To report suspected adverse drug events, for technical assistance or to obtain
a copy of the Safety Data Sheet (SDS), contact Huvepharma, Inc., at
1-877-994-4883 or www.huvepharma.us. For additional information about adverse
drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or
http://fda.gov/reportanimalae.
Net Weight: 50 lbs (22.68 kg)
Tylovet®100 Tylosin Phosphate
Directions for Use Read All Directions Carefully
Before Mixing and Feeding
Tylovet® 100 Type A Medicated Article Net Weight: 50 lbs
Do Not Feed Undiluted. (22.68 kg)
CAUTION:Federal law restricts medicated feed containing this veterinary feed directive (VFD)
drug to use by or on the order of a licensed veterinarian.
Active Drug Ingredient: Tylosin (as tylosin phosphate)...100 g per lb
Ingredients: Roughage products, pregelatanized starch and dipotassium phosphate.
Caution: To ensure adequate mixing, an intermediate blending step should be used prior to
manufacturing a complete feed. Do not use in any liquid feed containing sodium metabisulfite
or in any finished feed (supplement, concentrate or complete feed) containing in excess of 2%
bentonite.
Warning:Tylovet 100 may be irritating to unprotected skin and eyes. When mixing and handling
Tylovet 100 use protective clothing, impervious gloves and a dust respirator. In case of
accidental exposure, flush eyes with plenty of water. Exposed skin should be washed with plenty
of soap and water. Remove and wash contaminated clothing. Seek medical attention if irritation
becomes severe or persists. The safety data sheet (SDS) contains more detailed occupational
safety information. To report adverse effects, access medical information, or obtain additional
product information, call 1-877-994-4883.
For the reduction in severity of effects of atrophic rhinitis. Feed 100 g of tylosin per ton
(1.0 pound Tylovet 100 per ton) of complete feed. Feed continuously as the sole ration.
For control of swine dysentery. Feed 100 g of tylosin per ton (1.0 pound Tylovet 100 per ton)
of complete feed for at least three weeks. Follow with 40 g tylosin per ton (0.4 pounds
Tylovet 100 per ton) of complete feed until pigs reach market weight.
For the treatment and control of swine dysentery. Feed 40 to 100 grams of tylosin (0.4 to 1.0
pounds of Tylovet 100) per ton of complete feed for 2 to 6 weeks immediately after medicating
with 250 mg tylosin (as Tylosin Soluble) per gallon in drinking water for 3 to 10 days.
For the control of porcine proliferative enteropathies (PPE, ileitis). Feed 100 g tylosin per ton
(1.0 pound Tylovet 100 per ton) of complete feed for 21 days. Alternatively, feed 100 g of tylosin
per ton (1.0 pound Tylovet 100 per ton) of complete feed for at least three weeks, followed by
40 g tylosin per ton of complete feed until pigs reach market weight. Alternatively, feed 40 to 100
grams of tylosin (0.4 to 1.0 pounds of Tylovet 100) per ton of complete feed for 2 to 6 weeks
immediately after medicating with 250 mg tylosin (as Tylovet Soluble) per gallon in drinking water
for 3 to 10 days. Feed continuously as the sole ration when feeding Tylovet.
NOTICE: Organisms vary in their degree of susceptibility to any chemotherapeutic. If no improvement
is observed after recommended treatment, diagnosis and susceptibility should be reconfirmed.
|
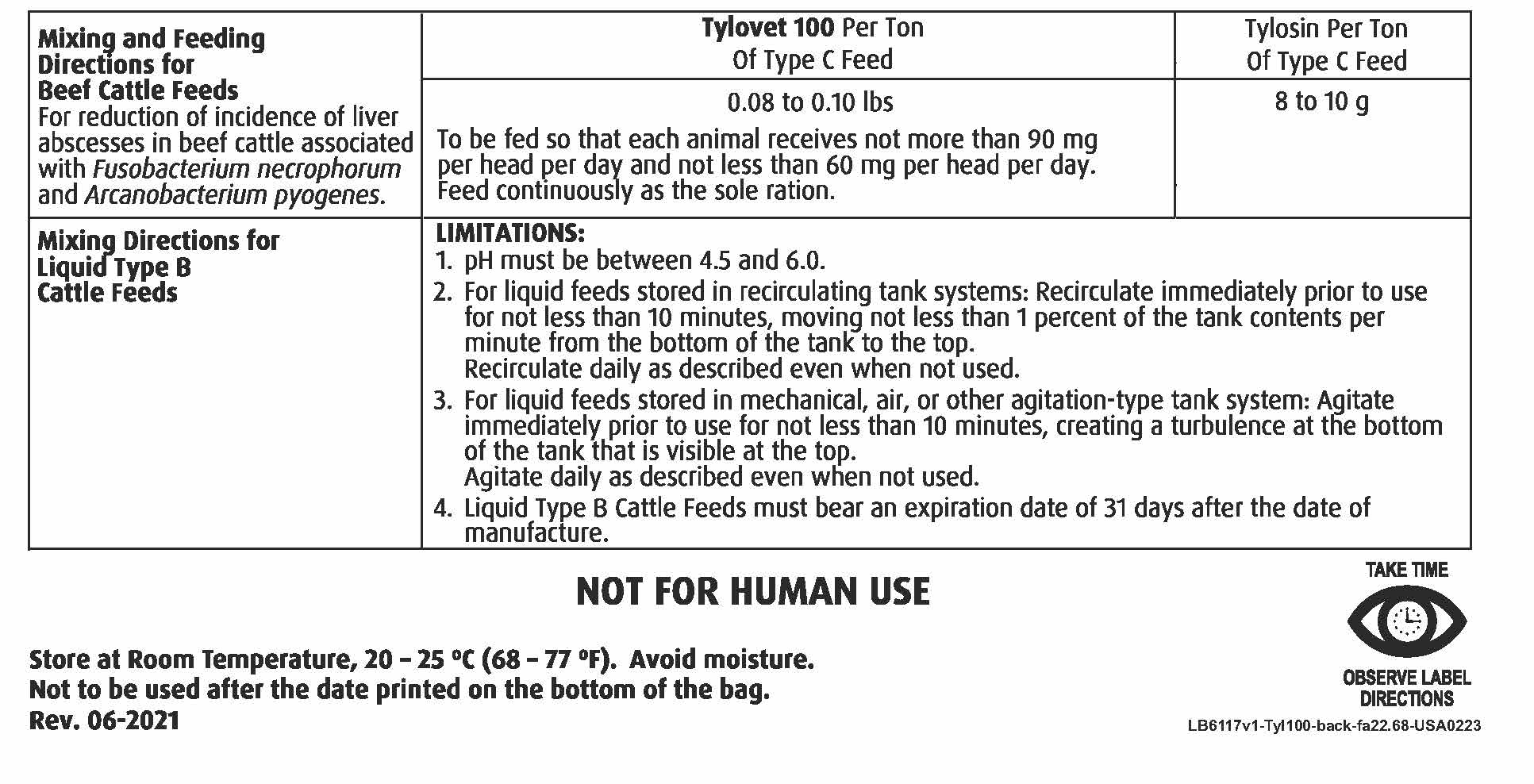
Mixing and Feeding Directions for Beef Cattle Feeds For reduction of incidence of liver abscesses associated with Fusobacterium necrophorum and Arcanobacterium pyogenes |
Tylovet 100 per Ton of Type C Feed |
Tylosin Per Ton of Type C Feed |
|
0.08 to 0.10 lbs To be fed so that each animal receives not more than 90 mg per head per day and not less than 60 mg per head per day. Feed continuously as the sole ration. |
8 to 10 g |
|
|
Mixing Directions for Liquid Type B Cattle Feeds |
LIMITATIONS: 1. pH must be between 4.5 and 6.0 2. For liquid feeds stored in recirculating tank systems: Recirculate immediately prior to use for not less than 10 minutes, moving not less than 1 percent of the tank contents per minute from the bottom of the tank to the top. Recirculate daily as described even when not used. 3. For liquid feeds stored in mechanical, air, or other agitation - type tank systems: Agitate immediately prior to use for not less than 10 minutes, creating a turbulence at the bottom of the tank that is visible at the top. Agitate daily as described even when not used. 4. Liquid Type B Cattle Feeds must bear an expiration date of 31 days after the date of manufacture. |
|