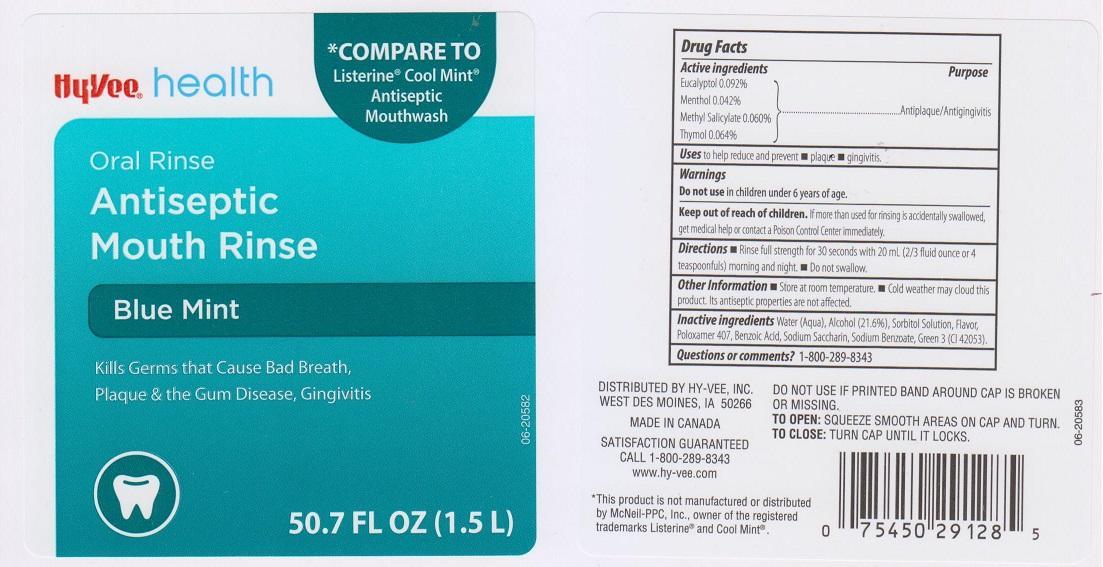
HYVEE ANTISEPTIC BLUE MINT- eucalyptol, menthol, methyl salicylate, thymol liquid
HYVEE INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Eucalyptol 0.092%
Menthol 0.042%
Methyl Salicylate 0.060%
Thymol 0.064%
Purpose
Antiplaque/Antigingivitis
Uses
to help reduce and prevent
Warnings
Do not use in children under 6 years of age
Keep out of reach of children.
If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Rinse full strength for 30 seconds with 20 mL (2/3 fluid ounce or 4 teaspoonfuls) morning and night
- Do not swallow
Other Information
- Store at room temperature.
- Cold weather may cloud this product. Its antiseptic properties are not affected.
Inactive ingredients
Water (Aqua), Alcohol (21.6%), Sorbitol Solution, Flavor, Poloxamer 407, Benzoic Acid, Sodium Saccharin, Sodium Benzoate, Green 3 (CI 42053)
Questions or comments?
1-800-289-8343
Label Copy