A1052-22 AMNIOCENTESIS 22G QUINCKE- sampler, amniotic fluid (amniocentesis tray)
Smiths Medical ASD, Inc.
----------
APLICARE POVIDONE-IODINE SOLUTION (povidone-iodine solution) solution
[Aplicare, Inc.]
3/4 Ounce Povidone Iodine Packet
Active Ingredient
Povidone-iodine
Purpose
Antiseptic
Use
Antispetic skin preparation
Warnings
Do not use
-if allergic to iodine.
-in the eyes
For external use only.
Ask a doctor before use if injuries are
-deep or puncture wounds
-serious burns
Stop use and ask a doctor if
-redness, irritation, swelling or pain persists or increases
-infection occurs
Keep out of reach of children
In case of accidental ingestion, seek professional help or consult a poison control center immediately.
Directions
Apply locally as needed.
Inactive Ingredients
citric acid, disodium phosphate, nonoxynol-9, sodium hydroxide, water
Questions or comments?
1 800 760-3236 (Mon to Fri 8:30 AM-5:00 PM EST)
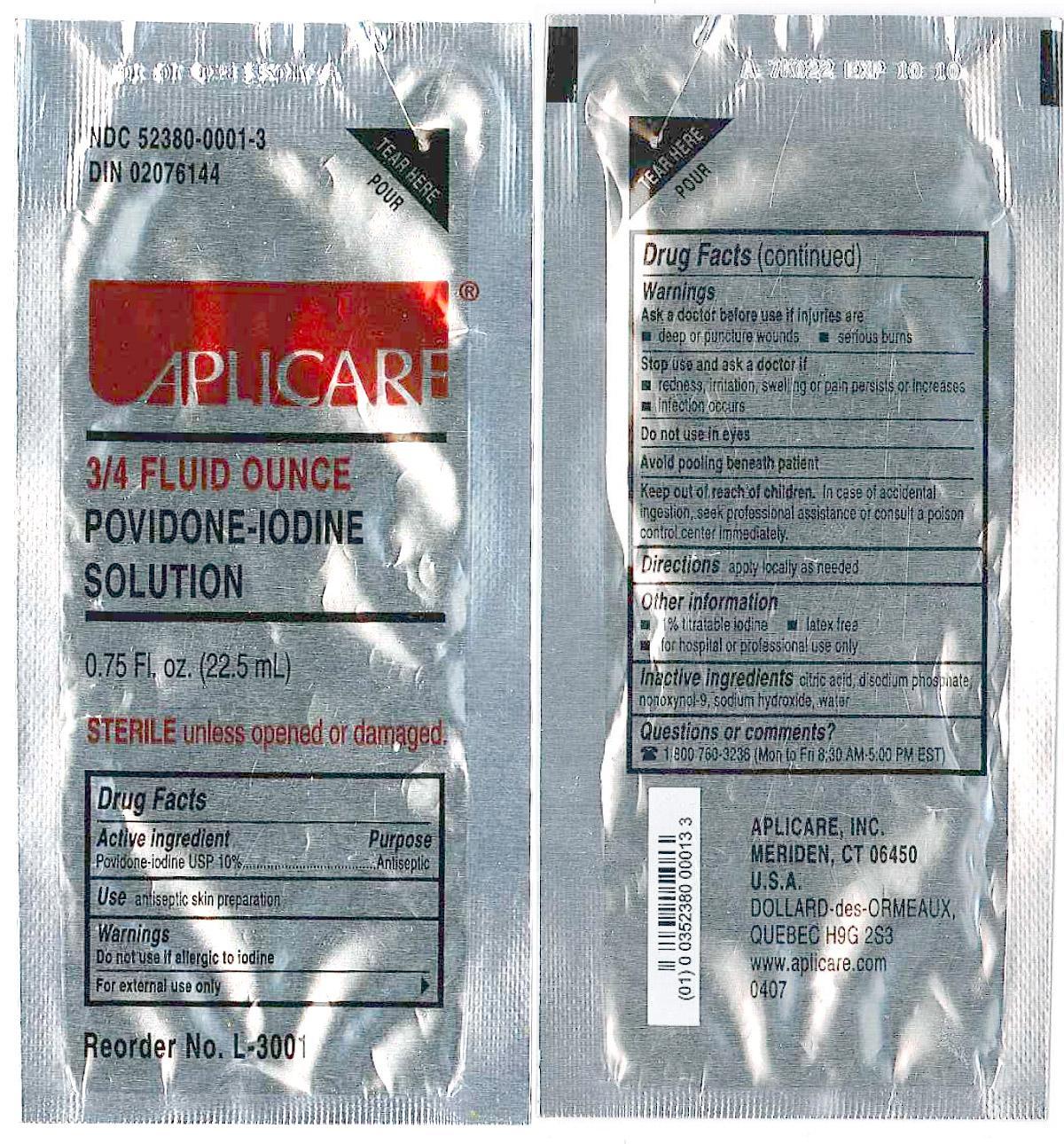
Package Label Display Panel

Smiths Medical ASD, Inc.

