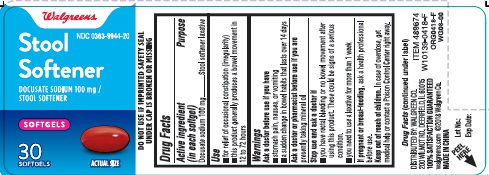
STOOL SOFTENER- docusate sodium capsule, liquid filled
Walgreen Co.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each softgel)
Docusate sodium 100 mg
Purpose
Stool softener laxative
Uses
- for relief of occasional constipation (irregularity)
- this product generally produces a bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain, nausea, or vomiting
- a sudden change in bowel habits that lasts over 14 days
Ask a doctor or pharmacist before use if you are presently taking mineral oil
Stop use and ask a doctor if
- you have rectal bleeding or no bowel movement after using this product. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
If pregnant or breast-feeding, ask a health professional before use
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
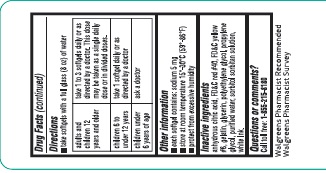
Directions
take softgels with a full glass (8oz) of water
|
adults and children 12 years and older
|
take 1 to 3 softgels daily or as directed by a doctor. This dose may be taken as a single daily dose or in divided doses.
|
|
children 6 to under 12 years
|
take 1 softgel daily or as directed by a doctor
|
|
children under 6 years
|
ask a doctor
|
Other information
- each softgel contains: sodium 5 mg
- store at room temperature 15°-30°C (59°-86°F)
- protect from excessive humidity
Inactive ingredients
anhydrous citric acid, FD&C red # 40, FD&C yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, purified water, sorbitol sorbitan solution and white edible ink
Questions or comments?
Call toll free: 1-855-215-8180
PRINCIPAL DISPLAY PANEL - Carton
Stool Softener
DOCUSATE SODIUM 100 mg 30 SOFTGELS
Compare to Phillips' Stool Softener Liquid Gels active ingredient
NDC 0363-9944-20


PRINCIPAL DISPLAY PANEL - Bottle
Stool Softener
DOCUSATE SODIUM 100 mg 30 SOFTGELS
Compare to Phillips' Stool Softener Liquid Gels active ingredient
NDC 0363-9944-20
Walgreen Co.



