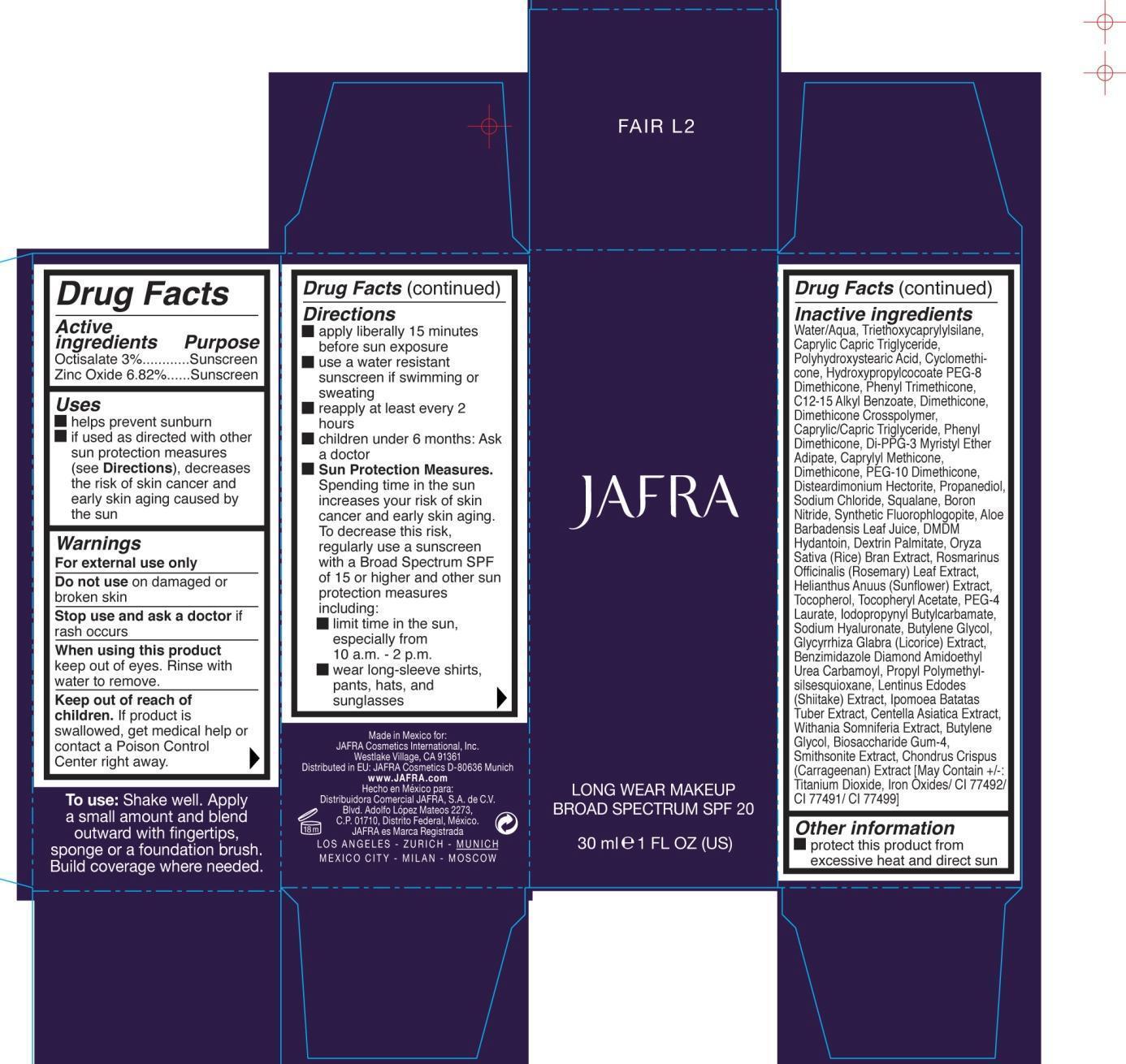
Uses
- helps prevent suburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Warnings
- For external use only
- Do not use on damaged or broken skin
- When using this product keep out of eyes. Rinse with water to remove.
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
Water/Aqua, Triethoxycaprylylsilane, Caprylic Capric Triglyceride, Polyhydroxystearic Acid, Cyclomethicone, Hydroxypropylcocoate PEG-8 Dimethicone, Phenyl Triemthicone, C12-15 Alkyl Benzoate, Dimethicone, Dimethicone Crosspolymer, Caprylic/Capric Triglyceride, Phenyl Dimethicone, Di-PPG-3 Myristyl Ether Adipate, Caprylyl Methicone, Dimethicone, PEG-10 Dimethicone, Disteardimonium Hectorite, Propanediol, Sodium Chloride, Squalane, Boron Nitride, Synthetic Fluorophlogopite, Aloe Barbadensis Leaf Juice, DMDM Hydantoin, Dextrin Palmitate, Oryza Sativa (Rice) Bran Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Helianthus Anuus (Sunflower) Extract, Tocopherol, Tocopheryl Acetate, PEG-4 Laurate, Iodopropynyl Butylcarbamate, Sodium Hyaluronate, Butylene Glycol, Glycyrrhiza Glabra (Licorice) Extract, Benzimidazole Diamond Amidoethyl Urea Carbamoyl Propyl Polymehtylsilsesquioxane, Lentinus Edodes (shiitake) Extract, Ipomoea Batatas Tuber Extract, Centella Asiatica Extract, Withania Somniferia Extract, Butylene Glycol, Biosaccharide Gum-4, Smithsonite Extact, Chondrus Crispus (Carrageenan) Extract [ May Containt +/-: Titanium Dioxide, Iron Oxides/ CI 77492/ CI 77491/ CI 77499]