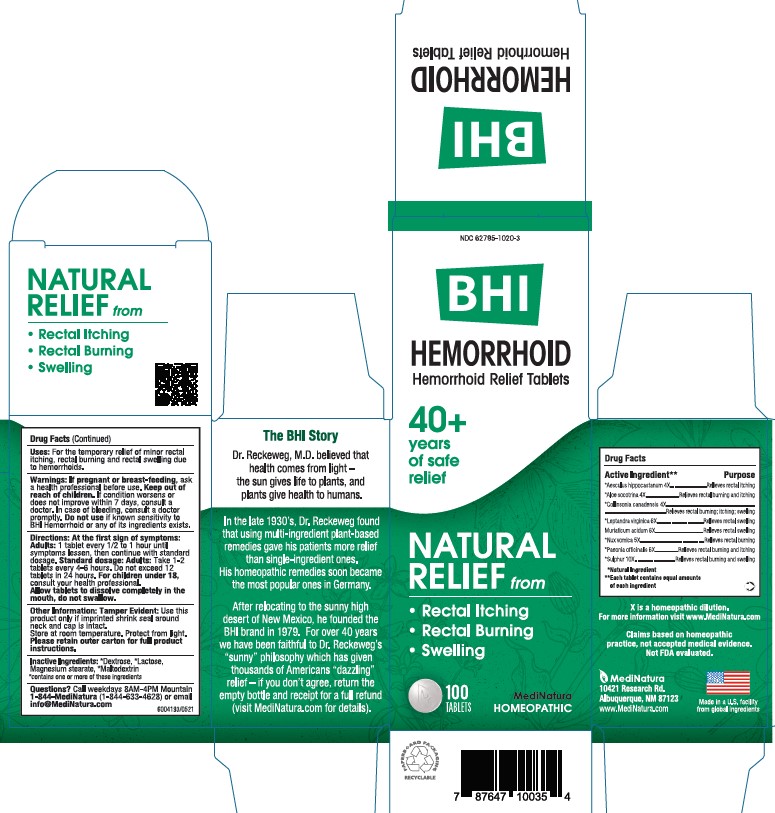
ACTIVE INGREDIENTS
Each tablet contains: *Aesculus hippocastanum 4X, *Aloe socotrina 4X, *Collinsonia canadensis 4X, *Leptandra virginica 6X, Muriaticum acidum 6X, *Nux vomica 5X, *Paeonia officinalis 6X, *Sulphur 10X 37.5 mg each.
*Natural Ingredients
INACTIVE INGREDIENTS
Inactive Ingredients: **Dextrose, ** Lactose, Magnesium Stearate, **Maltodextrin
**contains one or more of these ingredients.
USES
For the temporary relief of minor rectal itching, rectal burning, rectal swelling due to hemorrhoids
DIRECTIONS
At first sign of symptoms:
Adults: 1 tablet every 1/2 to 1 hour until symptoms lessen,
then continue with standard dosage.
Standard dosage: Adults: Take 1-2 tablets every 4 to 6 hours.
Do not exceed 12 tablets in 24 hours. For children under 18,
consult your health professional.
Allow tablets to dissolve completely in the mouth, do not swallow.
WARNINGS
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. If condition worsens or does not improve within 7 days, consult a doctor. In case of bleeding, consult a doctor promptly. Do not use if known sensitivity to Hemorrhoid or any of its ingredients exists.