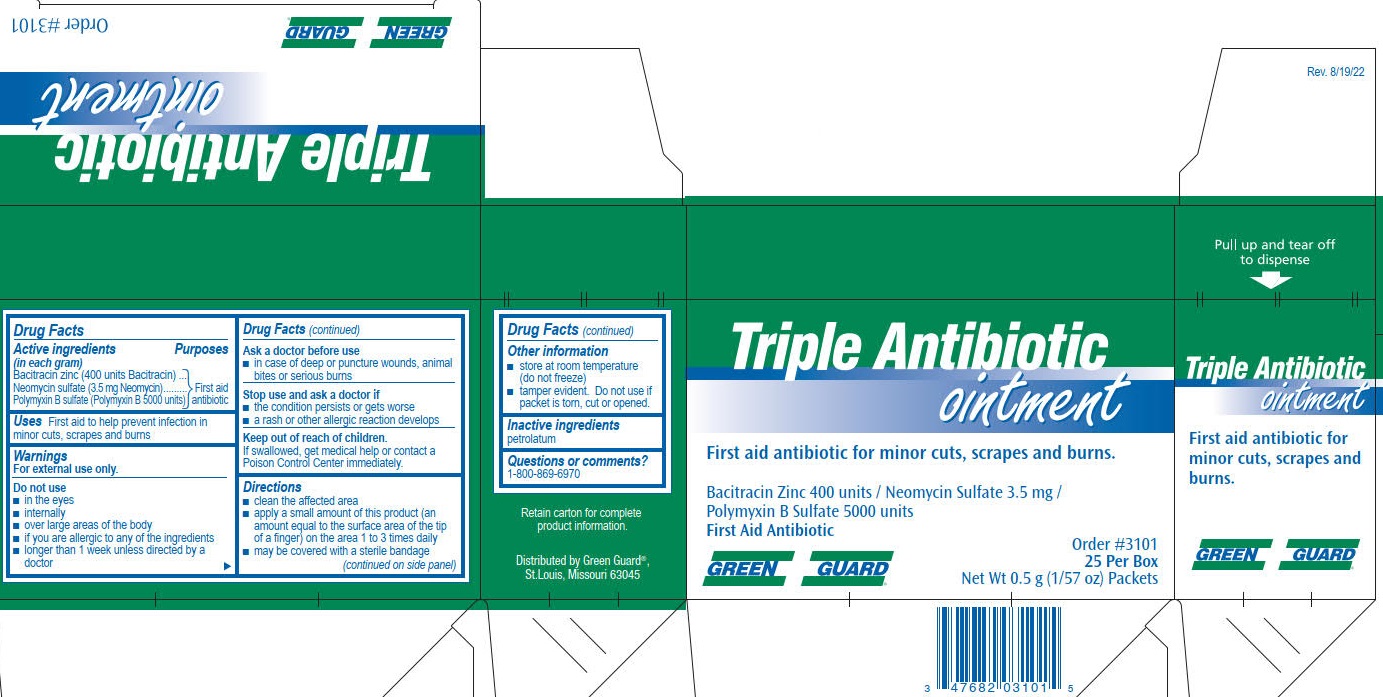
TRIPLE ANTIBIOTIC- bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointment
Unifirst First Aid Corporation
----------
Active Ingredients
(in each gram)
Bacitracin zinc (400 units Bacitracin)
Neomycin sulfate (3.5 mg Neomycin)
Polymyxin B sulfate (Polymyxin B 5000 units)
Purposes
First aid antibiotic
Uses
First aid to help prevent infection in minor cuts, scrapes and burns
Warnings
For external use only.
Do not use
- in the eyes
- internally
- over large areas of the body
- if you are allergic to any of the ingredients
- longer than 1 week unless directed by a doctor
Ask a doctor before use
- in case of deep puncture wounds, animal bites or serious burns
Stop use and ask a doctor if
- the condition persists or gets worse
- a rash or other allergic reaction develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- clean the affected area
- apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Other information
- store at romm temperature (do not freeze)
- tamper evident. Do not use if packet is torn, cut or opened.
Inactive ingredients
petrolatum
Questions or Comments? 1-800-869-6970
Green Guard Triple Antibiotic Ointment Label
Green Guard®
Triple Antibiotic
ointment
First aid antibiotic for minor cuts, scrapes and burns.
Bacitracin Zinc 4000 units/ Neomycin Sulfate 3.5 mg/ Polymyxin B Sulfate 5000 units
First Aid Antibiotic
Order #3101
25 Per Box
Net Wt 0.5 g (1/57 oz) Packets