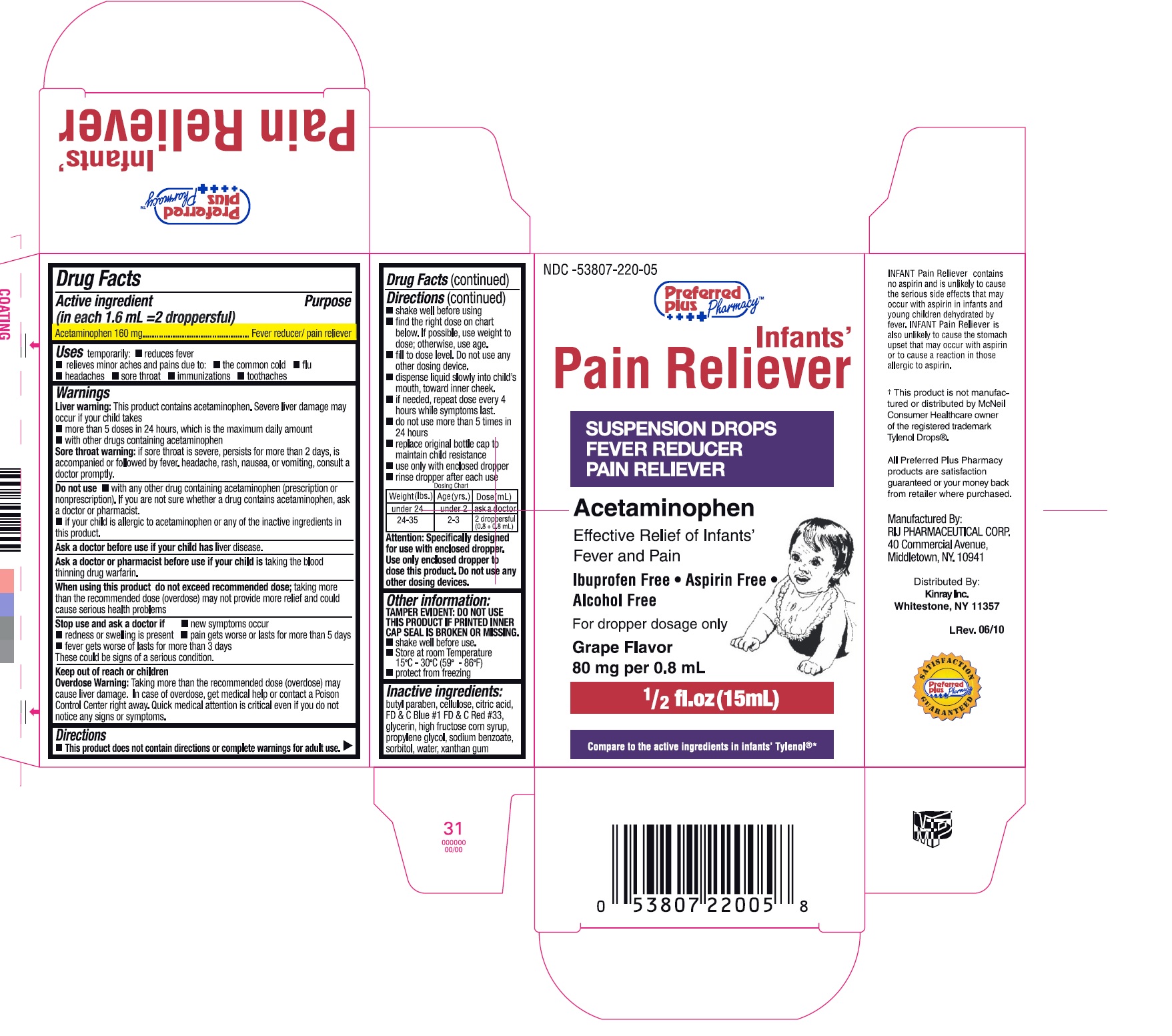
Uses
temporarily:
- reduces fever
- relieves minor aches and pains due to:
- the common cold
- flu
- headache
- sore throat
- immunizations
- toothache
Warnings
Liver warning:
This product contains acetaminophen.
Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Sore throat warning
if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
When using this product do not exceed recommended dose; taking more than the recommended dose (overdose) may not provide more relief and could cause serious health problems
Directions
- This product does not contain directions or complete warnings for adult use.
- shake well before using
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- fill to dose level. Do not use any other dosing device.
- dispense liquid slowly into child's mouth, toward inner cheek.
- if needed, repeat dose every 4 hours while symptoms last.
- do not give use than 5 times in 24 hours
- replace original bottle cap to maintain child resistance
- use only with enclosed dropper
- rinse dropper after each use
Dosing Chart
| Weight (lbs.) | Age (yrs.) | Dose ( mL) |
|---|---|---|
| under 24 | under 2 | ask a doctor |
| 24-35 | 2-3 | 2 droppersful (0.8 + 0.8 mL) |
Attention: Specifically designed for use with enclosed dropper. Use only enclosed dropper to dose this product. Do not use any other dosing devices.
Other information
- •
- TAMPER EVIDENT: DO NOT USE THIS PRODUCT IF PRINTED INNER CAP SEAL IS BROKEN OR MISSING
- •
- shake well before use.
- •
- Store at room Temperature 15º - 30ºC (59º - 86ºF)
- •
- protect from freezing.