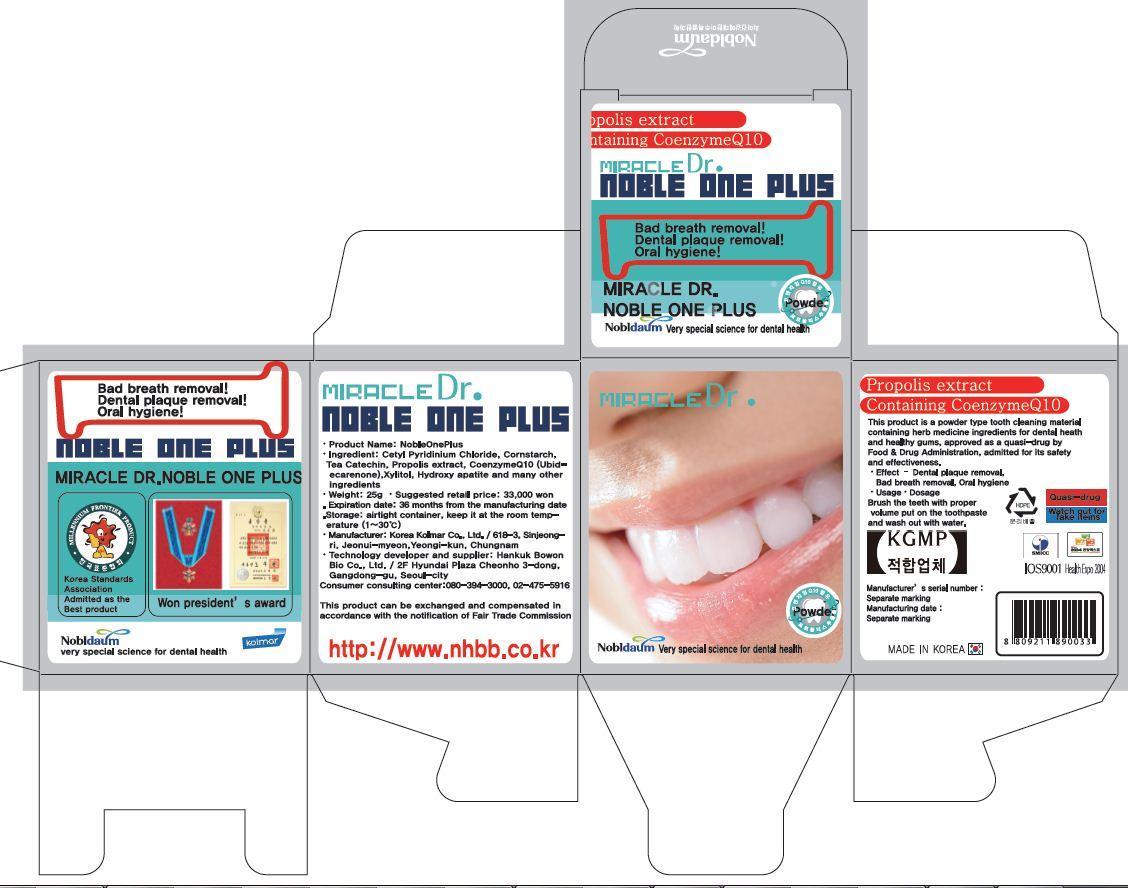
cetylpyridinium chloride, lactose monohydrate, corn starch, d-sorbitol, ubidecarenone, glycyrrhizae extract, tea chatechin, hydroxyapatite, propolis extract, menthol powder, spirulina color<text><contentstyleCode="xmChange"></content></text>
whiten and strong teeth
removal of bad breath
prevention of gingivitis and periodontitis
prevention of periodontal diseases and gum diseases
removal of dental plaque<text><contentstyleCode="xmChange"></content></text>
apply Proper Amount of the toothpaste on the tooth.<text><contentstyleCode="xmChange"></content></text>
■ For tooth only.
■ Avoid contact with eyes.
■ Do not swallow. If swallowed, get medical help.
<text><contentstyleCode="xmChange"></content></text>
 <text><contentstyleCode="xmChange"></content></text>
<text><contentstyleCode="xmChange"></content></text>