DESCRIPTION
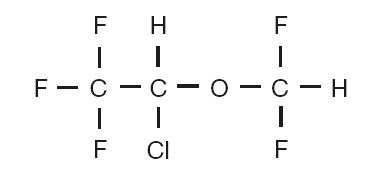
Isoflurane, USP, a nonflammable liquid administered by vaporizing, is a general inhalation anesthetic drug. It is 1-chloro-2,2,2-trifluoroethyl difluoromethyl ether, and its structural formula is:
Some physical constants are:
| Molecular weight | 184.5 | |
| Boiling point at 760 mm Hg | 48.5°C | |
Refractive index
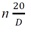 | 1.2990-1.3005 | |
| Specific gravity 25°/25°C | 1.496 | |
| Vapor pressure in mm Hg** | 20°C | 238 |
| 25°C | 295 | |
| 30°C | 367 | |
| 35°C | 450 |
**Equation for vapor pressure calculation:
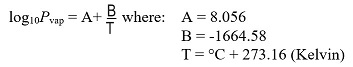
Partition coefficients at 37°C
| Water/gas | 0.61 |
| Blood/gas | 1.43 |
| Oil/gas | 90.8 |
| Partition coefficients at 25°C - rubber and plastic | ||
| Conductive rubber/gas | 62.0 | |
| Butyl rubber/gas | 75.0 | |
| Polyvinyl chloride/gas | 110.0 | |
| Polyethylene/gas | ~2.0 | |
| Polyurethane/gas | ~1.4 | |
| Polyolefin/gas | ~1.1 | |
| Butyl acetate/gas | ~2.5 | |
| Purity by gas chromatography | >99.9% | |
| Lower limit of flammability in oxygen or
nitrous oxide at 9 joules/sec. and 23°C | None | |
| Lower limit of flammability in oxygen or nitrous oxide at 900 joules/sec. and 23°C | Greater than useful concentration in anesthesia. | |
Isoflurane is a clear, colorless, stable liquid containing no additives or chemical stabilizers. Isoflurane has a mildly pungent, musty, ethereal odor. Samples stored in indirect sunlight in clear, colorless glass for five years, as well as samples directly exposed for 30 hours to a 2 amp, 115 volt, 60 cycle long wave U.V. light were unchanged in composition as determined by gas chromatography. Isoflurane in one normal sodium methoxide-methanol solution, a strong base, for over six months consumed essentially no alkali, indicative of strong base stability. Isoflurane does not decompose in the presence of soda lime (at normal operating temperatures), and does not attack aluminum, tin, brass, iron or copper.
CLINICAL PHARMACOLOGY
Induction of and recovery from isoflurane anesthesia are rapid. Isoflurane has a mild pungency which limits the rate of induction, although excessive salivation or tracheobronchial secretions do not appear to be stimulated. Pharyngeal and laryngeal reflexes are readily obtunded. The level of anesthesia may be changed rapidly with isoflurane. Isoflurane is a profound respiratory depressant. As anesthetic dose is increased, tidal volume decreases and respiratory rate is unchanged. This depression is partially reversed by surgical stimulation, even at deeper levels of anesthesia. Isoflurane evokes a sigh response reminiscent of that seen with diethyl ether and enflurane, although the frequency is less than with enflurane.
Blood pressure decreases with induction of anesthesia but returns toward normal with surgical stimulation. Progressive increases in depth of anesthesia produce corresponding decreases in blood pressure. Nitrous oxide diminishes the inspiratory concentration of isoflurane required to reach a desired level of anesthesia and may reduce the arterial hypotension seen with isoflurane alone. Heart rhythm is remarkably stable. With controlled ventilation and normal PaCO2, cardiac output is maintained despite increasing depth of anesthesia, primarily through an increase in heart rate which compensates for a reduction in stroke volume. The hypercapnia which attends spontaneous ventilation during isoflurane anesthesia further increases heart rate and raises cardiac output above awake levels.
Muscle relaxation is often adequate for intra-abdominal operations at normal levels of anesthesia. Complete muscle paralysis can be attained with small doses of muscle relaxants. ALL COMMONLY USED MUSCLE RELAXANTS ARE MARKEDLY POTENTIATED WITH ISOFLURANE, THE EFFECT BEING MOST PROFOUND WITH THE NONDEPOLARIZING TYPE. Neostigmine reverses the effect of nondepolarizing muscle relaxants in the presence of isoflurane. All commonly used muscle relaxants are compatible with isoflurane.
Isoflurane can produce coronary vasodilation at the arteriolar level in selected animal models; the drug is probably also a coronary dilator in humans. Isoflurane, like some other coronary arteriolar dilators, has been shown to divert blood from collateral dependent myocardium to normally perfused areas in an animal model (“coronary steal”). Clinical studies to date evaluating myocardial ischemia, infarction and death as outcome parameters have not established that the coronary arteriolar dilation property of isoflurane is associated with coronary steal or myocardial ischemia in patients with coronary artery disease.
Pharmacokinetics: Isoflurane undergoes minimal biotransformation in man. In the postanesthesia period, only 0.17% of the isoflurane taken up can be recovered as urinary metabolites.
Pharmacogenomics:RYR1 and CACNA1S are polymorphic genes, and multiple pathogenic variants have been associated with malignant hyperthermia susceptibility (MHS) in patients receiving volatile anesthetic agents, including isoflurane. Case reports as well as ex-vivo studies have identified multiple variants in RYR1 and CACNA1S associated with MHS. Variant pathogenicity should be assessed based on prior clinical experience, functional studies, prevalence information, or other evidence (see CONTRAINDICATIONS, WARNINGS).
INDICATIONS AND USAGE
Isoflurane may be used for induction and maintenance of general anesthesia. Adequate data have not been developed to establish its application in obstetrical anesthesia.
CONTRAINDICATIONS
Isoflurane is contraindicated in patients:
• in whom general anesthesia is contraindicated.
• with known sensitivity to isoflurane or to other halogenated agents.
• with known or suspected genetic susceptibility to malignant hyperthermia (see WARNINGS/Malignant Hyperthermia, CLINICAL PHARMACOLOGY/ Pharmacogenomics).
• with a history of confirmed hepatitis due to a halogenated inhalational anesthetic or a history of unexplained moderate to severe hepatic dysfunction (e.g., jaundice associated with fever and/or eosinophilia) after anesthesia with isoflurane or other halogenated inhalational anesthetics.
WARNINGS
Perioperative Hyperkalemia: Use of inhaled anesthetic agents has been associated with rare increases in serum potassium levels that have resulted in cardiac arrhythmias and death in pediatric patients during the postoperative period. Patients with latent as well as overt neuromuscular disease, particularly Duchenne muscular dystrophy, appear to be most vulnerable. Concomitant use of succinylcholine has been associated with most, but not all, of these cases. These patients also experienced significant elevations in serum creatinine kinase levels and, in some cases, changes in urine consistent with myoglobinuria. Despite the similarity in presentation to malignant hyperthermia, none of these patients exhibited signs or symptoms of muscle rigidity or hypermetabolic state. Early and aggressive intervention to treat the hyperkalemia and resistant arrhythmias is recommended, as is subsequent evaluation for latent neuromuscular disease.
Malignant Hyperthermia: In susceptible individuals, volatile anesthetic agents, including isoflurane, may trigger malignant hyperthermia, a skeletal muscle hypermetabolic state leading to high oxygen demand. Fatal outcomes of malignant hyperthermia have been reported.
The risk of developing malignant hyperthermia increases with the concomitant administration of succinylcholine and volatile anesthetic agents. Isoflurane can induce malignant hyperthermia in patients with known or suspected susceptibility based on genetic factors or family history, including those with certain inherited ryanodine receptor ( RYR1) or dihydropyridine receptor ( CACNA1S) variants. [see CONTRAINDICATIONS, CLINICAL PHARMACOLOGY/Pharmacogenomics]
Signs consistent with malignant hyperthermia may include hyperthermia, hypoxia, hypercapnia, muscle rigidity (e.g., jaw muscle spasm), tachycardia (e.g., particularly that unresponsive to deepening anesthesia or analgesic medication administration), tachypnea, cyanosis, arrhythmias, hypovolemia, and hemodynamic instability. Skin mottling, coagulopathies, and renal failure may occur later in the course of the hypermetabolic process.
Successful treatment of malignant hyperthermia depends on early recognition of the clinical signs. If malignant hyperthermia is suspected, discontinue all triggering agents (i.e., volatile anesthetic agents and succinylcholine), administer intravenous dantrolene sodium, and initiate supportive therapies. Consult prescribing information for intravenous dantrolene sodium for additional information on patient management. Supportive therapies include administration of supplemental oxygen and respiratory support based on clinical need, maintenance of hemodynamic stability and adequate urinary output, management of fluid and electrolyte balance, correction of acid base derangements, and institution of measures to control rising temperature.
Hepatic Reactions
Cases of mild, moderate and severe postoperative hepatic dysfunction or hepatitis with or without jaundice, including fatal hepatic necrosis and hepatic failure, have been reported with isoflurane.
Such reactions can represent hypersensitivity hepatitis, a known risk of exposure to halogenated anesthetics, including isoflurane. As with other halogenated anesthetic agents, Isoflurane may cause sensitivity hepatitis in patients who have been sensitized by previous exposure to halogenated anesthetics (see CONTRAINDICATIONS).
Clinical judgment should be exercised when isoflurane is used in patients with underlying hepatic conditions or under treatment with drugs known to cause hepatic dysfunction. (see CONTRAINDICATIONS).
As with all halogenated anesthetics, repeated anesthetics within a short period of time may result in increased effects, particularly in patients with underlying hepatic conditions, or additive effects in patients treated with drugs known to cause hepatic dysfunction. Evaluate the need for repeated exposure in each individual patient and adjust the dose of isoflurane based on signs and symptoms of adequate depth of anesthesia if repeated exposure in a short period of time is clinically indicated.
Hypersensitivity Reactions
Allergic-type hypersensitivity reactions, including anaphylaxis, have been reported with isoflurane. Manifestations of such reactions have included hypotension, rash, difficulty breathing and cardiovascular collapse
Abortions
Increased blood loss comparable to that seen with halothane has been observed in patients undergoing abortions.
QTc Prolongation
QTc prolongation, with rare instances of torsade de pointes, have been reported. Monitor QT interval when administering isoflurane to susceptible patients.
Pediatric Neurotoxicity: Published animal studies demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity increase neuronal apoptosis in the developing brain and result in long-term cognitive deficits when used for longer than 3 hours. The clinical significance of these findings is not clear. However, based on the available data, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately three years of age in humans. (see PRECAUTIONS/Pregnancy, Pediatric Use, and ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY).
Some published studies in children suggest that similar deficits may occur after repeated or prolonged exposures to anesthetic agents early in life and may result in adverse cognitive or behavioral effects. These studies have substantial limitations, and it is not clear if the observed effects are due to the anesthetic/sedation drug administration or other factors such as the surgery or underlying illness.
Anesthetic and sedation drugs are a necessary part of the care of children needing surgery, other procedures, or tests that cannot be delayed, and no specific medications have been shown to be safer than any other. Decisions regarding the timing of any elective procedures requiring anesthesia should take into consideration the benefits of the procedure weighed against the potential risks.
PRECAUTIONS
General
As with any potent general anesthetic, isoflurane should only be administered in an adequately equipped anesthetizing environment by those who are familiar with the pharmacology of the drug and qualified by training and experience to manage the anesthetized patient.
All patients anesthetized with isoflurane should be continually monitored (e.g., monitoring of the electrocardiogram, blood pressure, oxygen saturation, and end tidal CO 2). Isoflurane is a profound respiratory depressant. Excessive respiratory depression may be related to depth of anesthesia and respond to decreasing the inspired concentration of isoflurane. The depressant effect is accentuated by concurrent use of opioids and other respiratory depressants. Respiration should be closely monitored and assisted or controlled ventilation employed when necessary.
With the exception of neonates, isoflurane MAC decreases with increasing age.
Regardless of the anesthetics employed, maintenance of normal hemodynamics is important to the avoidance of myocardial ischemia in patients with coronary artery disease.
Isoflurane can cause dose-dependent coronary vasodilation and has been shown to divert blood from collateral-dependent myocardium to normally perfused areas in an animal model (“coronary steal”). The extent to which coronary steal occurs in patients with steal- prone coronary anatomy is unclear. Monitor for signs of inadequate myocardial perfusion via hemodynamic monitors (e.g., ECG, blood pressure) during isoflurane administration. Consider additional cardiac monitoring in patients with known coronary artery disease, as clinically necessary.
Isoflurane causes a dose-dependent reduction in systemic vascular resistance and blood pressure. Particular care must be taken when selecting the dosage for patients who are hypovolemic, hypotensive, or otherwise hemodynamically compromised, e.g., due to concomitant medications.
Isoflurane markedly increases cerebral blood flow at deeper levels of anesthesia to produce a transient increase in intracranial pressure. In patients with or at risk for elevations of intracranial pressure (ICP), administer isoflurane in conjunction with ICP-reducing strategies, as clinically appropriate.
Isoflurane, like some other inhalational anesthetics, can react with desiccated carbon dioxide (CO 2) absorbents to produce carbon monoxide, which may result in elevated levels of carboxyhemoglobin in some patients. Barium hydroxide lime and soda lime become desiccated when fresh gases are passed through the CO 2 absorber canister at high flow rates over many hours or days. When a clinician suspects that CO 2 absorbent may be desiccated, it should be replaced before the administration of isoflurane.
The color indicator of most CO 2 absorbents does not necessarily change as a result of desiccation. Therefore, the lack of significant color change should not be taken as assurance of adequate hydration of the CO 2 absorbent material. CO 2 absorbents should be replaced routinely regardless of the state of color indicator following current manufacturer’s guidelines for use of anesthesiology equipment.
The following reactions have been reported following occupational exposure to isoflurane: dyspnea, bronchospasm, stridor, cough, dizziness, paresthesia, hepatic reactions, flushing rash, contact dermatitis, erythema, periorbital edema, eye irritation, conjunctival hyperemia, and headache.
Information for Patients
Isoflurane, as well as other general anesthetics, may cause a slight decrease in intellectual function for 2 or 3 days following anesthesia. As with other anesthetics, small changes in moods and symptoms may persist for up to 6 days after administration.
Effect of anesthetic and sedation drugs on early brain development: Studies conducted in young animals and children suggest repeated or prolonged use of general anesthetic or sedation drugs in children younger than 3 years may have negative effects on their developing brains. Discuss with parents and caregivers the benefits, risks, and timing and duration of surgery or procedures requiring anesthetic and sedation drugs (see WARNINGS/Pediatric Neurotoxicity).
Laboratory Tests
Transient increases in BSP retention, blood glucose and serum creatinine with decrease in BUN, serum cholesterol and alkaline phosphatase have been observed.
Drug Interactions
Opioids decrease the Minimum Alveolar Concentration (MAC) of isoflurane. Opioids such as fentanyl and its analogues, when combined with isoflurane, may lead to a synergistic fall in blood pressure and respiratory rate.
Nitrous oxide decreases the MAC of isoflurane (see DOSAGE AND ADMINISTRATION).
Isoflurane potentiates the muscle relaxant effect of all muscle relaxants and decreases the required doses of neuromuscular blocking agents. In general, anesthetic concentrations of isoflurane at equilibrium reduce the ED 95 of succinylcholine, atracurium, pancuronium, rocuronium and vecuronium by approximately 25 to 40% or more compared to N 2O/opioid anesthesia. If added relaxation is required, supplemental doses of muscle relaxants may be used.
Isoflurane is similar to sevoflurane in the sensitization of the myocardium to arrhythmogenic effect of exogenously administered adrenaline. Doses of adrenaline greater than 5mcg/kg, when administered submucosally may produce multiple ventricular arrhythmias. Isoflurane may lead to marked hypotension in patients treated with calcium antagonists.
Concomitant use of beta blockers may exaggerate the cardiovascular effects of inhalational anesthetics, including hypotension and negative inotropic effects.
Concomitant use of MAO inhibitors and inhalational anesthetics may increase the risk of hemodynamic instability during surgery or medical procedures.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis
Swiss ICR mice were given isoflurane to determine whether such exposure might induce neoplasia. Isoflurane was given at 1/2, 1/8 and 1/32 MAC for four in-utero exposures and for 24 exposures to the pups during the first nine weeks of life. The mice were killed at 15 months of age. The incidence of tumors in these mice was the same as in untreated control mice, which were given the same background gases, but not the anesthetic.
Mutagenesis
Isoflurane was negative in the in vivo mouse micronucleus and in vitro human lymphocyte chromosomal aberration assay. In published studies, isoflurane was negative in the in vitro bacterial reverse mutation assay (Ames test) in all strains tested ( Salmonella typhimurium strains TA98, TA100, and TA1535) in the presence or absence of metabolic activation.
Impairment of Fertility
Male and female Sprague-Dawley rats were exposed to isoflurane at concentrations of 0%, 0.15%, and 0.60% (0, 1/8, and 1/2 MAC) 2 hours per day for 14 consecutive days prior to mating. Isoflurane had no effects on either male or female fertility.
Pregnancy
Risk Summary
There are no adequate and well-controlled studies in pregnant women. In animal reproduction studies, embryofetal toxicity was noted in pregnant mice exposed to 0.075% (increased post implantation losses) and 0.3% isoflurane (increased post implantation losses and decreased livebirth index) during organogenesis.
Published studies in pregnant primates demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity during the period of peak brain development increases neuronal apoptosis in the developing brain of the offspring when used for longer than 3 hours. There are no data on pregnancy exposures in primates corresponding to periods prior to the third trimester in humans [See Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Pregnant rats were exposed to isoflurane at concentrations of 0%, 0.1%, or 0.4% for two hours per day during organogenesis (Gestational Days 6-15). Isoflurane did not cause malformations or clear maternal toxicity under these conditions.
Pregnant mice exposed to isoflurane at concentrations of 0%, 0.075%, or 0.30% for 2 hours per day during organogenesis (Gestational Days 6-15). Isoflurane increased fetal toxicity (higher post implantation losses at 0.075 and 0.3% groups and significantly lower live-birth index in the 0.3% isoflurane treatment group). Isoflurane did not cause malformations or clear maternal toxicity under these conditions.
Pregnant rats were exposed to concentrations of isoflurane at 0%, 0.1%, or 0.4% for 2 hours per day during late gestation (GD 15-20). Animals appeared slightly sedated during exposure. No adverse effects on the offspring or evidence of maternal toxicity were reported. This study did not evaluate neurobehavioral function including learning and memory in the first generation (F1) of pups.
In a published study in primates, administration of an anesthetic dose of ketamine for 24 hours on Gestation Day 122 increased neuronal apoptosis in the developing brain of the fetus. In other published studies, administration of either isoflurane or propofol for 5 hours on Gestation Day 120 resulted in increased neuronal and oligodendrocyte apoptosis in the developing brain of the offspring. With respect to brain development, this time period corresponds to the third trimester of gestation in the human. The clinical significance of these findings is not clear; however, studies in juvenile animals suggest neuroapoptosis correlates with long-term cognitive deficits. (See WARNINGS/Pediatric Neurotoxicity, PRECAUTIONS/Pediatric Use, and ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY).
Nursing Mothers
Due to insufficient information regarding the excretion of isoflurane in human milk, the potential risks and benefits for each specific patient should be carefully considered before isoflurane is administered to nursing women.
Pediatric Use
During the induction of anesthesia, saliva flow and tracheobronchial secretion can increase and can be the cause of larynogospasm, particularly in children.
Published juvenile animal studies demonstrate that the administration of anesthetic and sedation drugs, such as Isoflurane, that either block NMDA receptors or potentiate the activity of GABA during the period of rapid brain growth or synaptogenesis, results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester of gestation through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of ketamine that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer of isoflurane increased neuronal cell loss. Data from isoflurane-treated rodents and ketamine-treated primates suggest that the neuronal and oligodendrocyte cell losses are associated with prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in pregnant women, neonates, and young children who require procedures with the potential risks suggested by the nonclinical data (see WARNINGS/Pediatric Neurotoxicity, PRECAUTIONS/Pregnancy, and ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY).
Effects on Ability to Drive and Use Machines
Patients should be advised that performance of activities requiring mental alertness, such as driving a vehicle or operating machinery, may be impaired for some time after general anesthesia. Therefore, patients should not undertake hazardous tasks, such as driving, for at least 24 hours following administration of a general anesthetic.
ADVERSE REACTIONS
The following adverse reactions were identified from controlled clinical studies of adult and pediatric subjects exposed to isoflurane. The studies were conducted using a variety of pre-medications, other anesthetics, and surgical procedures of varying lengths.
The most serious reported adverse reactions in alphabetical order are agitation, arrhythmia, breath holding, elevated liver enzyme, hypotension and laryngospasm.
The most frequent adverse reactions (incidence ≥ 5%) described in Table 1 are agitation, breath holding, chills/shivering, cough, delirium, laryngospasm, nausea, and vomiting.
Adverse reactions with and incidence between 1% and 5% are provided in Table 2. Adverse reactions with an incidence less than 1% are provided in Table 3.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Table 1: Adverse Reactions ≥ 5%
|
System Organ Class (SOC) |
Adverse Reaction |
Frequency |
|
|
PSYCHIATRIC DISORDERS |
Delirium |
6.2% (N=2830) |
|
|
NERVOUS SYSTEM DISORDERS |
Agitation (Excitement) |
Induction |
51.8% (N=515) 1 |
|
RESPIRATORY, THORACIC, AND MEDIASTINAL DISORDERS |
Breath holding |
Induction |
23.9% (N=515) 1 |
|
Cough |
Induction |
28.2% (N=515) 1 |
|
|
Laryngospasm |
Induction |
8.0% (N=515) 1 |
|
|
GASTROINTESTINAL DISORDERS |
Nausea |
Recovery |
15.4 % (N=2830) |
|
Vomiting |
Recovery |
9.5% (N=2830) |
|
|
GENERAL DISORDERS AND ADMINISTRATIVE SITE CONDITIONS |
Chills/shivering |
14.0% (N=1691) 2 |
|
1 Represents patients not receiving intravenous agents or muscle relaxants for intubation (i.e., patients receiving inhalation induction).
2 Reflects the number of patients with recorded body temperature measurements.
Table 2: Adverse Reactions between 1% and 5%
|
System Organ Class (SOC) |
Adverse Reaction |
Frequency |
|
|
NERVOUS SYSTEM DISORDERS |
Movement |
Maintenance |
1.8% N=2830) |
| CARDIAC DISORDERS |
Ventricular arrhythmia (Intraoperative) |
Induction |
2.1% (N=2161) |
|
Maintenance |
2.7% (N=2253) |
||
|
Nodal arrhythmia (Intraoperative) |
Induction |
4.0% (N=2161) |
|
|
Maintenance |
1.7% (N=2253) |
||
|
Atrial arrhythmia (Intraoperative) |
Induction |
1.6% (N=2161) |
|
|
Maintenance |
2.2% (N=2253) |
||
|
Arrhythmia (Postoperative) |
1.1% (N=2830) |
||
|
RESPIRATORY, THORACIC, AND MEDIASTINAL DISORDERS |
Breath holding |
Maintenance |
1.1% (N=359) 1 |
|
Cough |
Maintenance |
4.2 % (N=359) 1 |
|
1 Represents patients not receiving intravenous agents or muscle relaxants for intubation (i.e., patients receiving inhalation induction).
Table 3: Adverse Reactions less than 1%
|
System Organ Class (SOC) |
Adverse Reaction |
Frequency |
|
|
PSYCHIATRIC DISORDERS |
Mood changes |
0.3% (N=2830) |
|
|
Nightmare |
0.4% (N=2175) 1 |
||
|
NERVOUS SYSTEM DISORDERS |
Convulsive pattern on electroencephalogram |
0.5% (N=200) 2 |
|
|
Seizure |
0.04% (N=2830) |
||
|
VASCULAR DISORDERS |
Hypotension |
Postoperative |
0.3% (N=2830) |
|
Hypertension |
Postoperative |
0.1% (N=2830) |
|
|
RESPIRATORY, THORACIC, AND MEDIASTINAL DISORDERS |
Laryngospasm |
Maintenance |
0.8% (N=359) 3 |
|
Secretions |
Induction |
0.2% (N=515) 3 |
|
|
Maintenance |
0.0% (N=359) 3 |
||
|
GASTROINTESTINAL DISORDERS |
Vomiting |
Induction |
0.8% (N=515) 3 |
|
Retching |
Induction |
1.0% (N=515) 3 |
|
|
Maintenance |
0.8% (N=359) 3 |
||
|
SKIN AND SUBCUTANEOUS TISSUE DISORDERS |
Diaphoresis |
Induction |
0.2% (N=515) 3 |
|
Maintenance |
0.0% (N=359) 3 |
||
1 Reflects the number of patients interviewed by a physician in the recovery period.
2 Reflects the number of recorded electroencephalograms.
3 Represents patients not receiving intravenous agents or muscle relaxants for intubation (i.e., patients receiving inhalation induction).
The following adverse reactions were observed, but due to limited data, frequency could not be determined.
|
Blood and Lymphatic System Disorders: |
White blood cell count increased |
|
Metabolism and Nutrition Disorders: |
Blood glucose increased |
|
Psychiatric Disorders: |
Confused state, Nervousness |
|
Nervous System Disorders: |
Ataxia; Dizziness; Drowsiness; Intellectual function decrease |
|
Vascular Disorders: |
Hypotension (Intraoperative); Hypertension (Intraoperative) |
|
Hepatobiliary Disorders: |
Blood bilirubin increased; Bromsulphthalein clearance decreased; Alanine aminotransferase increased; Aspartate aminotransferase increased; Blood alkaline phosphatase increased; Blood lactate dehydrogenase increased |
|
Musculoskeletal, Connective Tissue and Bone Disorders: |
Myalgia |
|
General Disorders and Administrative Site Conditions: |
Asthenia; Fatigue |
Post-Marketing Adverse Reactions
The following adverse reactions have been reported in the post-marketing experience, listed by MedDRA System Organ Class (SOC), then by preferred term in order of decreasing severity.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
BLOOD AND LYMPHATIC SYSTEM DISORDERS: Carboxyhemoglobin increased
IMMUNE SYSTEM DISORDERS: Anaphylactic reaction
METABOLISM AND NUTRITION DISORDERS: Hyperkalemia in patients with underlying myopathies
PSYCHIATRIC DISORDERS: Withdrawal syndrome (following multi-day exposure; symptoms include seizure, hallucination, ataxia, agitation, confusion )
NERVOUS SYSTEM DISORDERS: Brain edema, Intracranial pressure increased, Migraine, Myoclonus, Nystagmus, Pupils unequal, Headache
CARDIAC DISORDERS: Cardiac arrest, Ventricular fibrillation, Torsade de pointes, Myocardial infarction, Myocardial ischemia, Atrioventricular block complete, Atrioventricular block second degree, Atrial fibrillation, Electrocardiogram QT prolonged, Atrioventricular block first degree, Ventricular tachycardia, Ventricular extrasystoles, Tachycardia, Bradycardia, Cardiac output decreased.
VASCULAR DISORDERS: Flushing
RESPIRATORY, THORACIC, AND MEDIASTINAL DISORDERS: Apnea, Hypoxia, Bronchospasm, Airway obstruction, Respiratory depression, Hypercapnia, Stridor, Hiccough
GASTROINTESTINAL DISORDERS: Pancreatitis
HEPATOBILIARY DISORDERS: Hepatic necrosis, Hepatic failure, Hepatitis fulminant, Cholestatic hepatitis, Hepatitis, Hepatic steatosis, Jaundice, Gamma- glutamyltransferase increased.
SKIN AND SUBCUTANEOUS TISSUE DISORDERS: Rash
MUSCULOSKELETAL, CONNECTIVE TISSUE AND BONE DISORDERS: Rhabdomyolysis
RENAL AND URINARY DISORDERS: Acute renal failure**, Oliguria**
GENERAL DISORDERS AND ADMINISTRATION SITE CONDITIONS: Malignant hyperthermia, hypothermia
INJURY, POISONING, AND PROCEDURAL COMPLICATIONS*: Unwanted awareness during anesthesia; Dyspnea, Bronchospasm, Stridor, Cough, Dizziness, Paresthesia, Hepatic reactions, Flushing, Rash, Contact dermatitis, Erythema, Periorbital edema, Eye irritation, Conjunctival hyperemia, Headache
*All reactions categorized within this SOC, with the exception of, Unwanted awareness during anesthesia, were from occupational exposure in non-patients.
**Cases of acute renal failure and oliguria have been reported after isoflurane anesthesia. These events may be secondary to hypotension or other effects of isoflurane.
OVERDOSAGE
In the event of overdosage, or what may appear to be overdosage, the following action should be taken, as appropriate:
Stop drug administration, establish a clear airway, and initiate assisted or controlled ventilation with pure oxygen. Monitor cardiovascular function and manage signs of poor end-organ perfusion as clinically indicated.
DOSAGE & ADMINISTRATION
Isoflurane should be administered only by persons trained in the administration of general anesthesia.
Facilities for maintenance of a patent airway, artificial ventilation, oxygen enrichment, and circulatory resuscitation must be immediately available.
Isoflurane is administered by inhalation. Isoflurane should be delivered from a vaporizer specifically designed for use with isoflurane.
The minimum alveolar concentration (MAC) of isoflurane decreases with increasing patient age.
Dosage for induction and maintenance must be individualized and titrated to the desired effect according to the patient’s age and clinical status.
Premedication: Premedication should be selected according to the need of the individual patient, taking into account that secretions are weakly stimulated by isoflurane, and the heart rate tends to be increased.
Induction: Induction with isoflurane in oxygen or in combination with oxygen-nitrous oxide mixtures may produce coughing, breath holding, or laryngospasm and bronchospasm, which increases with the concentration of isoflurane. These difficulties may be avoided by the use of a hypnotic dose of an ultra-short-acting barbiturate. Inspired concentrations of 1.5 to 3.0% isoflurane usually produce surgical anesthesia in 7 to 10 minutes.
Maintenance: Isoflurane MAC values according to age are shown below:
|
Age |
Average MAC Value In 100% Oxygen |
Average MAC Value In 30% Oxygen and 70% N 2 O |
|
Preterm neonates ˂32 weeks gestational age |
1.28% | |
|
Preterm neonates 32-37 weeks gestational age |
1.41% | |
|
0-1 month |
1.60% | |
|
1-6 months |
1.87% | |
|
6-12 months |
1.80% | |
|
1-5 years |
1.60% | |
|
6-10 years |
1.45% | |
|
11-18 years |
1.38% | |
|
19-30 years |
1.28% |
0.56% |
|
31-55 years |
1.15% |
0.50% |
|
55-83 years |
1.05% |
0.37% |
Dosage for induction and maintenance must be individualized and titrated to the desired effect according to the patient’s age and clinical status.
Surgical levels of anesthesia may be sustained with a 1.0 to 2.5% concentration when nitrous oxide is used concomitantly. An additional 0.5 to 1.0% may be required when isoflurane is given using oxygen alone. If added relaxation is required, supplemental doses of muscle relaxants may be used.
The level of blood pressure during maintenance is an inverse function of isoflurane concentration in the absence of other complicating problems. Excessive decreases may be due to depth of anesthesia and in such instances may be corrected by lightening anesthesia.
HOW SUPPLIED
Isoflurane, USP is packaged in 100 mL and 250 mL amber-colored bottles.
100 mL - NDC 66794-017-10
250 mL - NDC 66794-017-25
Safety and Handling
OCCUPATIONAL CAUTION
There is no specific work exposure limit established for Isoflurane. However, the National Institute for Occupational Safety and Health Administration (NIOSH) recommends that no worker should be exposed at ceiling concentrations greater than 2 ppm of any halogenated anesthetic agent over a sampling period not to exceed one hour.
The predicted effects of acute overexposure by inhalation of Isoflurane include headache, dizziness or (in extreme cases) unconsciousness. There are no documented adverse effects of chronic exposure to halogenated anesthetic vapors ( Waste Anesthetic Gases or WAGs) in the workplace. Although results of some epidemiological studies suggest a link between exposure to halogenated anesthetics and increased health problems (particularly spontaneous abortion), the relationship is not conclusive. Since exposure to WAGs is one possible factor in the findings for these studies, operating room personnel, and pregnant women in particular, should minimize exposure. Precautions include adequate general ventilation in the operating room, the use of a well-designed and well-maintained scavenging system, work practices to minimize leaks and spills while the anesthetic agent is in use, and routine equipment maintenance to minimize leaks (See PRECAUTIONS).
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Preserve in tight containers. Isoflurane contains no additives and has been demonstrated to be stable at room temperature for periods in excess of five years.
ANIMAL TOXICOLOGY AND/OR PHARMACOLOGY
Published studies in animals demonstrate that the use of anesthetic agents during the period of rapid brain growth or synaptogenesis results in widespread neuronal and oligodendrocyte cell loss in the developing brain and alterations in synaptic morphology and neurogenesis. Based on comparisons across species, the window of vulnerability to these changes is believed to correlate with exposures in the third trimester through the first several months of life, but may extend out to approximately 3 years of age in humans.
In primates, exposure to 3 hours of an anesthetic regimen that produced a light surgical plane of anesthesia did not increase neuronal cell loss, however, treatment regimens of 5 hours or longer increased neuronal cell loss. Data in rodents and in primates suggest that the neuronal and oligodendrocyte cell losses are associated with subtle but prolonged cognitive deficits in learning and memory. The clinical significance of these nonclinical findings is not known, and healthcare providers should balance the benefits of appropriate anesthesia in neonates and young children who require procedures against the potential risks suggested by the nonclinical data (see WARNINGS/Pediatric Neurotoxicity and PRECAUTIONS/Pregnancy, Pediatric Use).
Manufactured By:
Piramal Pharma Limited
Sy. Nos. 7-70, 70/1 & 70/2, Digwal Village,
Kohir Mandal, SANGAREDDY District,
Telangana State, India
Revised: December 2022