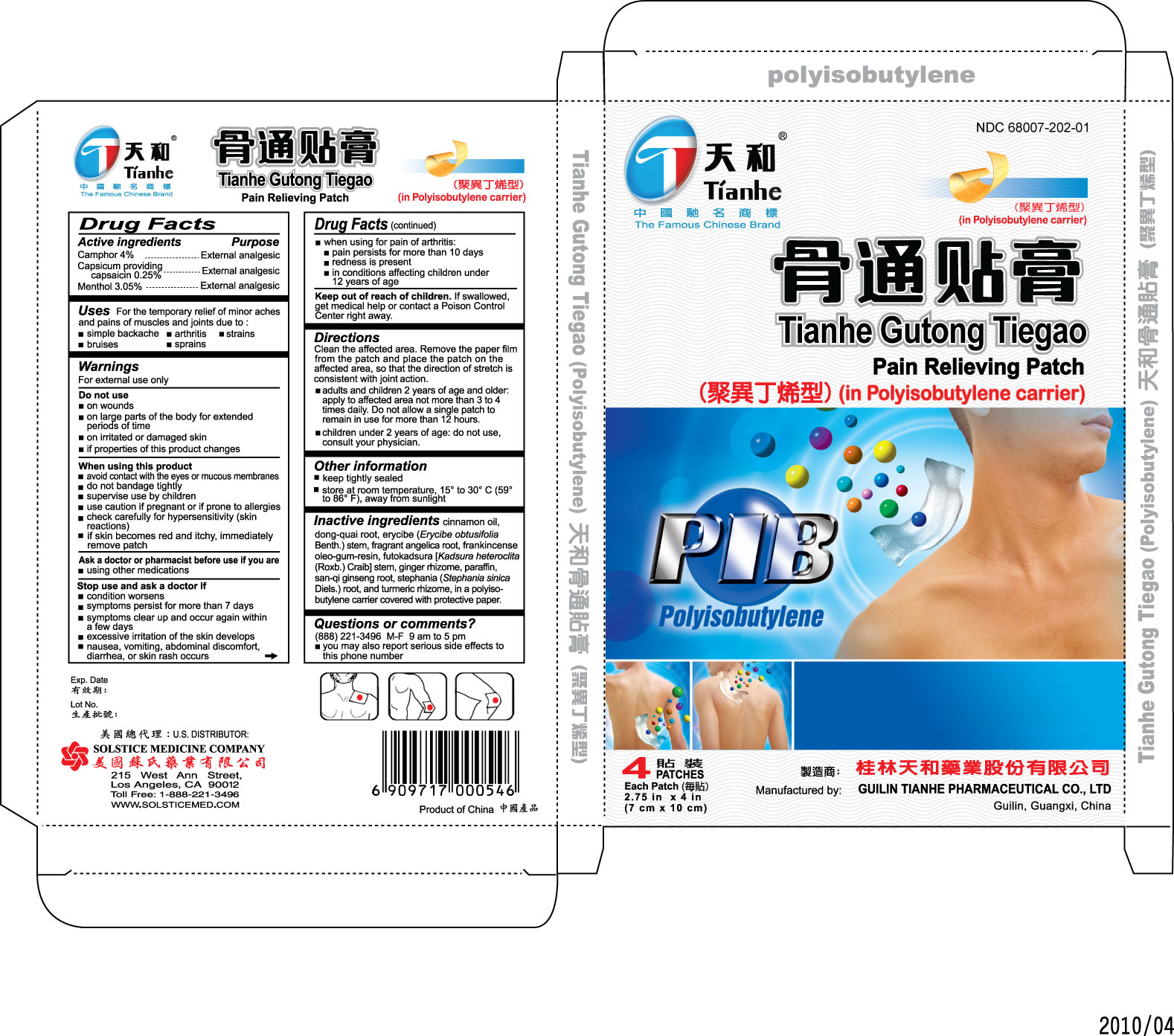
Purpose
External analgesic
Uses For the temporary relief of minor aches and pains of muscles and joints due to:
- simple backache
- arthritis
- strains
- bruises
- sprains
Do not use
- on wounds
- on large parts of the body for extended periods of time
- on irritated or damaged skin
- if properties of this product changes
When using this product
- avoid contact with the eyes or mucous membranes
- do not bandage tightly
- supervise use by children
- use caution if pregnant or if prone to allergies
- check carefully for hypersensitivity (skin reactions)
- if skin becomes red and or itchy, immediately remove patch
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive irritation of the skin develops
- nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
- when using for pain of arthritis:
-
- pain persists for more than 10 days
- redness is present in conditions affecting children under 12 years of age
- pain persists for more than 10 days
Keep out of reach of children. If swallowed, get medical help or contact a
Poison Control Center right away.
Directions Clean the affected area. Remove the paper film from the patch and place the patch on the affected area, so that the direction of stretch is consistent with joint action.
- adults and children 2 years of age and older: apply to affected area no more than 3 to 4 times daily. Do not allow a single patch to remain in use for more than 12 hours.
- children under 2 years of age: do not use, consult your physician.
Other information
- keep tightly sealed
- store at room temperature, 15º to 30º C (59º to 86º F), away from sunlight
Inactive ingredients cinnamon oil, dong-quai root, erycibe (Erycibe
obtusifolia Benth.) stem, fragrant angelica root, frankincense oleo-gum-resin,
futokadsura [Kadsura heteroclita (Roxb.) Craib] stem, ginger rhizome, paraffin,
san-qi ginseng root, stephania (Stephania sinica Diels.) root, and turmeric
rhizome, in a polyisobutylene carrier covered with protective paper.