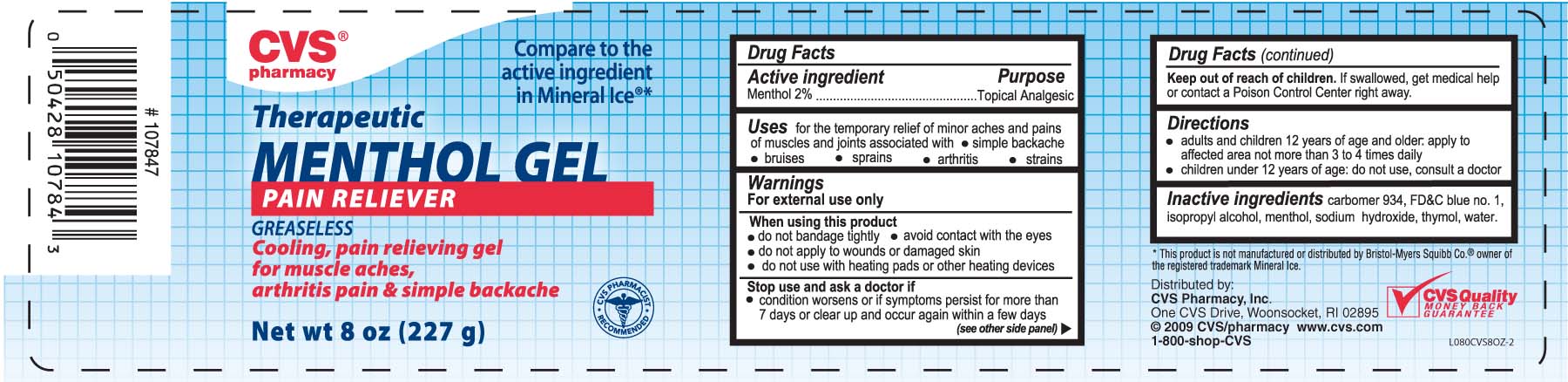
Active ingredient Purpose
Menthol 2%...................................................Topical Analgesic
Menthol 2%...................................................Topical Analgesic
Uses for the temporary relief of minor aches and pains of muscles and joints
associated with - simple backache - bruises - sprains - arthritis - strains
Keep out of reach of children. If swallowed, get medical help or contact a
Poison Control Center right away.
Uses for the temporary relief of minor aches and pains of muscles and joints
associated with - simple backache - bruises - sprains - arthritis - strains
Warnings
For external use only
When using this product
- do not bandage tightly - avoid contact with the eyes
- do not apply to wounds or damaged skin
- do not use with heating pads or other heating devices
Stop use and ask a doctor if - condition worsens or if symptoms persist
for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.If swallowed, get medical help or contact a
Poison Control Center right away.
For external use only
When using this product
- do not bandage tightly - avoid contact with the eyes
- do not apply to wounds or damaged skin
- do not use with heating pads or other heating devices
Stop use and ask a doctor if - condition worsens or if symptoms persist
for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.If swallowed, get medical help or contact a
Poison Control Center right away.
Directions
- adults and children 12 years of age and older, apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: do not use, consult a doctor