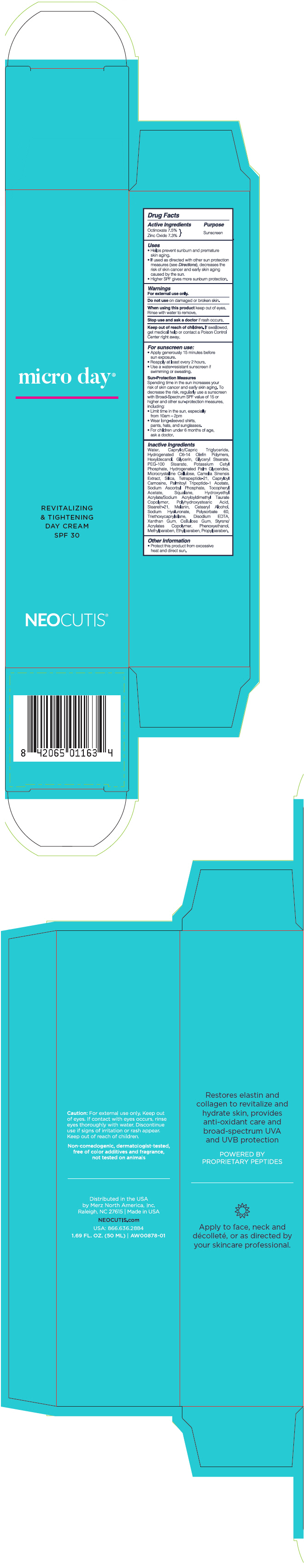
Uses
- Helps prevent sunburn and premature skin aging.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Higher SPF gives more sunburn protection.
Warnings
For external use only.
For sunscreen use:
- Apply generously 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with Broad-Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10am - 2pm
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Children under 6 months of age, ask a doctor.
Inactive ingredients
Water, Caprylic/Capric Triglyceride, Hydrogenated C6-14 Olefin Polymers, Hexyldecanol, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Potassium Cetyl Phosphate, Hydrogenated Palm Glycerides, Microcrystalline Cellulose, Camelia Sinensis Extract, Silica, Tetrapeptide-21, Capryloyl Carnosine, Palmitoyl Tripeptide-1 Acetate, Sodium Ascorbyl Phosphate, Tocopheryl Acetate, Squalane, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Polyhydroxystearic Acid, Steareth-21, Melanin, Cetearyl Alcohol, Sodium Hyaluronate, Polysorbate 60, Triethoxycaprylsilane, Disodium EDTA, Xanthan Gum, Cellulose Gum, Styrene/Acrylates Copolymer, Phenoxyethanol, Methylparaben, Ethylparaben, Propylparaben.