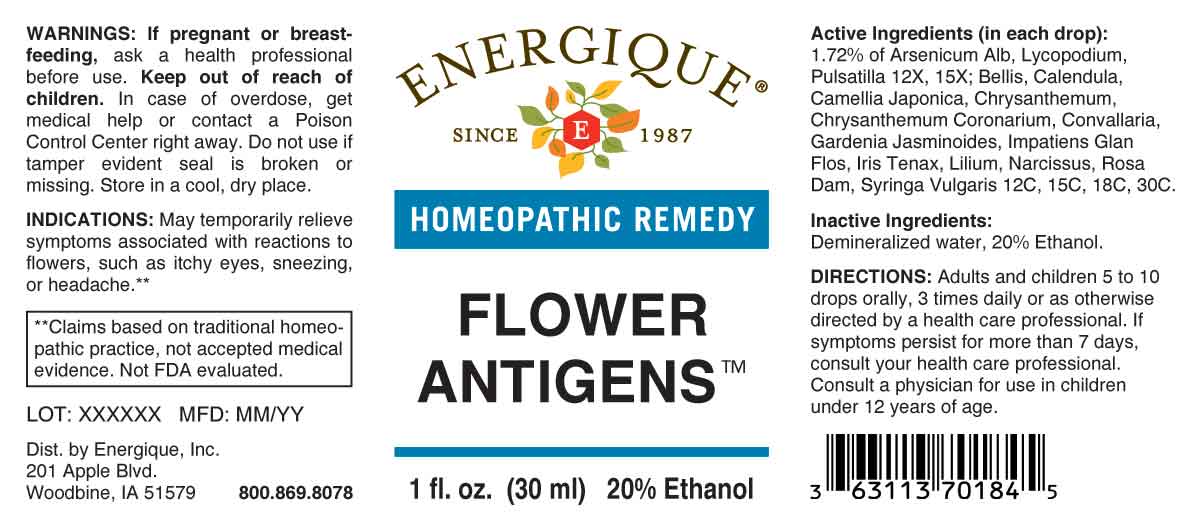
ACTIVE INGREDIENTS:
(in each drop): 1.72% of Arsenicum Album 12X, 15X, Lycopodium Clavatum 12X, 15X, Pulsatilla 12X, 15X; Bellis Perennis 12C, 15C, 18C, 30C, Calendula Officinalis 12C, 15C, 18C, 30C, Camellia Japonica 12C, 15C, 18C, 30C, Chrysanthemum Coronarium 12C, 15C, 18C, 30C, Chrysanthemum Leucanthemum 12C, 15C, 18C, 30C, Convallaria Majalis 12C, 15C, 18C, 30C, Gardenia Jasminoides 12C, 15C, 18C, 30C, Impatiens Glandulifera, Flos 12C, 15C, 18C, 30C, Iris Tenax 12C, 15C, 18C, 30C, Lilium Tigrinum 12C, 15C, 18C, 30C, Narcissus Pseudo-Narcissus 12C, 15C, 18C, 30C, Rosa Damascena 12C, 15C, 18C, 30C, Syringa Vulgaris 12C, 15C, 18C, 30C.
INDICATIONS:
May temporarily relieve symptoms associated with reactions to flowers, such as itchy eyes, sneezing, or headache.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS:
Adults and children 5 to 10 drops orally, 3 times daily or as otherwise directed by a health care professional. If symptoms persist for more than 7 days, consult your health care professional. Consult a physician for use in children under 12 years of age.