Warnings
Do not use
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
When using this product
- avoid alcoholic drinks
- drowsiness will occur
- do not drive a motor vehicle or operate machinery
Overdose warning: In case of overdose get medical help or contact a Poison Control Center right away.
Other information
- each caplet contains: calcium 20 mg
- store between 20-25°C (68-77°F)
- do not use if carton is opened or blister unit is broken
- see side panel for lot number and expiration date
Inactive ingredients
carnauba wax, cellulose, croscarmellose sodium dibasic calcium phosphate, FD&C blue #1, hypromellose, magnesium stearate, polyethylene glycol, polysorbate 80, titanium dioxide
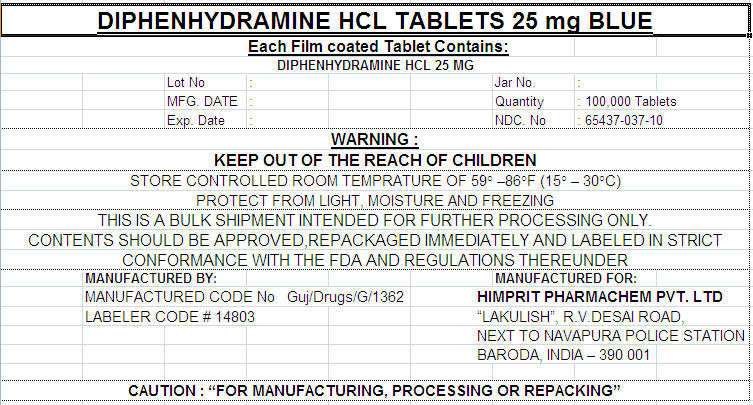
PRINCIPAL DISPLAY PANEL - 25 mg Label
DIPHENHYDRAMINE HCL TABLETS 25 mg BLUE
Each Film coated Tablet Contains:
DIPHENHYDRAMINE HCL 25 MG
| Lot No | : | Jar No. | : | ||
| MFG. DATE | : | Quantity | : 100,000 Tablets | ||
| Exp. Date | : | NDC. No | : 65437-037-10 |
WARNING :
KEEP OUT OF THE REACH OF CHILDREN
STORE CONTROLLED ROOM TEMPRATURE OF 59° –86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZING
THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED,REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE FDA AND REGULATIONS THEREUNDER
MANUFACTURED BY:
MANUFACTURED CODE No Guj/Drugs/G/1362
LABELER CODE # 14803
MANUFACTURED FOR:
HIMPRIT PHARMACHEM PVT. LTD
"LAKULISH", R.V.DESAI ROAD,
NEXT TO NAVAPURA POLICE STATION
BARODA, INDIA – 390 001
CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"