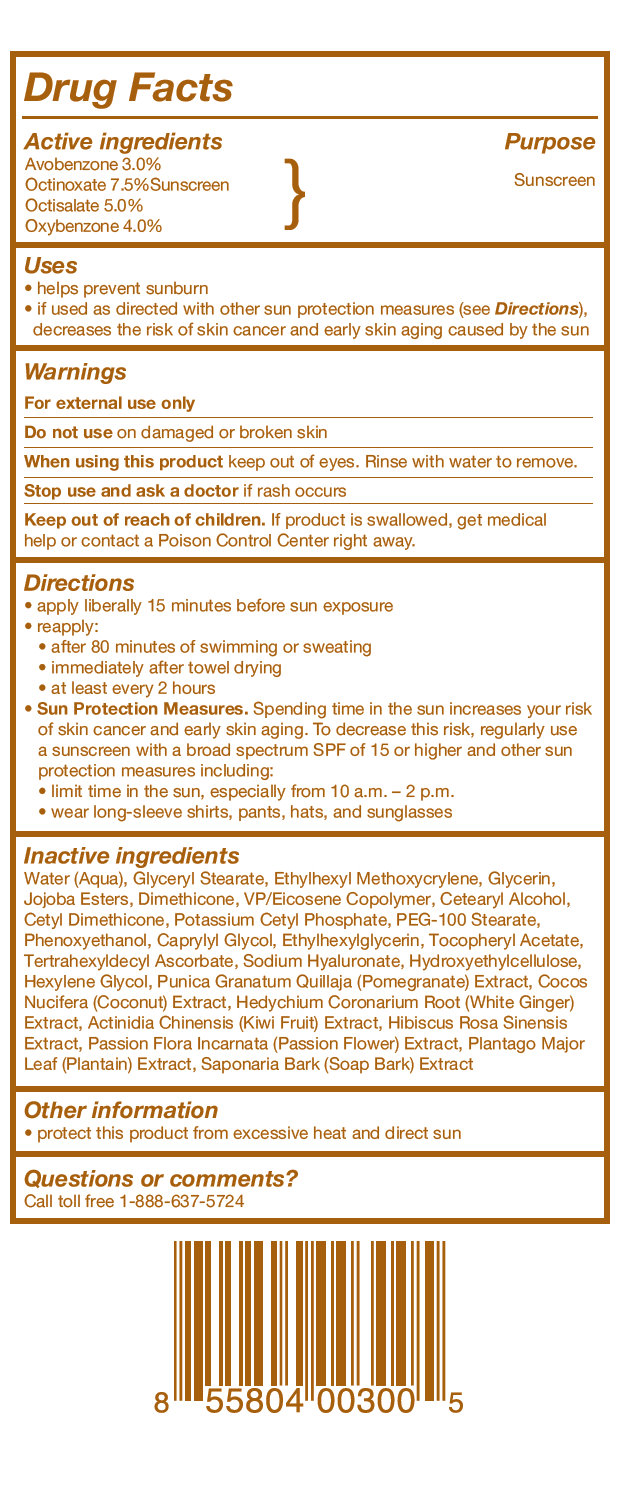
Active ingredients
Active ingredients Purpose
Avobenzone 3.0%
Octinoxate 7.5%Sunscreen } Sunscreen
Octisalate 5.0%
Oxybenzone 4.0%
Uses
• helps prevent sunburn
• if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Keep out of reach of children
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
• apply liberally 15 minutes before sun exposure
• reapply:
• after 80 minutes of swimming or sweating
• immediately after towel drying
• at least every 2 hours
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
• limit time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeve shirts, pants, hats, and sunglasses.
Inactive ingredients
Water (Aqua), Glyceryl Stearate, Ethylhexyl Methoxycrylene, Glycerin, Jojoba Esters, Dimethicone, VP/Eicosene Copolymer, Cetearyl Alcohol, Cetyl Dimethicone, Potassium Cetyl Phosphate, PEG-100 Stearate, Phenoxyethanol, Caprylyl Glycol, Ethylhexylglycerin, Tocopheryl Acetate, Tertrahexyldecyl Ascorbate, Sodium Hyaluronate, Hydroxyethylcellulose, Hexylene Glycol, Punica Granatum Quillaja (Pomegranate) Extract, Cocos Nucifera (Coconut) Extract, Hedychium Coronarium Root (White Ginger) Extract, Actinidia Chinensis (Kiwi Fruit) Extract, Hibiscus Rosa Sinensis Extract, Passion Flora Incarnata (Passion Flower) Extract, Plantago Major Leaf (Plantain) Extract, Saponaria Bark (Soap Bark) Extract