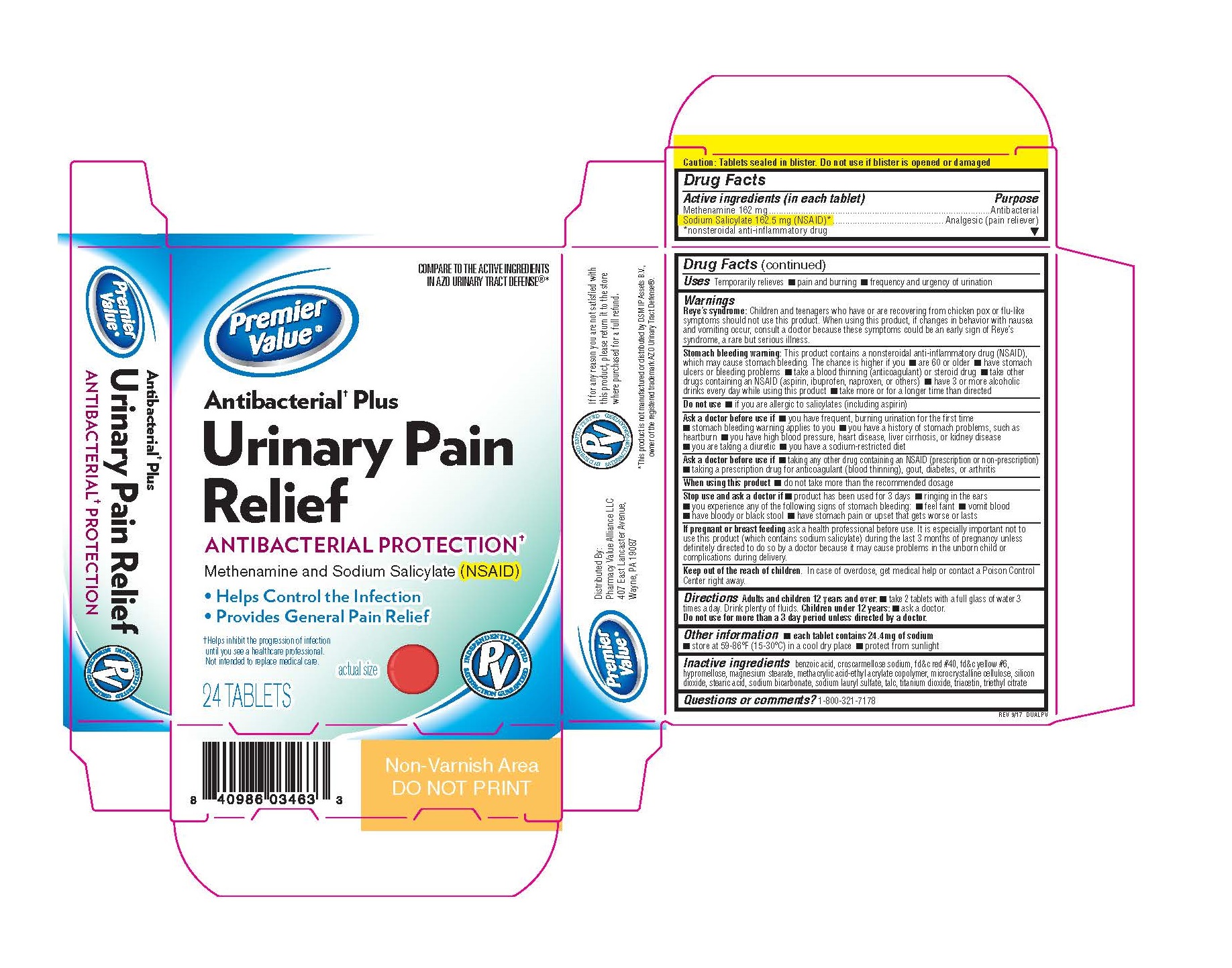
Active Ingredients (in each tablet):
Methenamine 162 mg
Sodium Salicylate 162.5 mg (NSAID Nonsteroidal Anti-Inflammatory Drug)
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. If changes in behaviour with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Stomach bleeding warning: This product contains an NSAID, which may cause stomach bleeding. The chance is higher if you:
• take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen or others)
• have 3 or more alcoholic drinks every day while using this product
• have stomach ulcers or bleeding problems • take a blood thinning (anticoagulant) or steroid drug
• are age 60 or older • take more or for a longer time than directed
Do not use:
• if you are on a sodium restricted diet
• if you are allergic to salicylates (including aspirin) unless directed by a doctor
• if you have stomach problems (such as heartburn, upset stomach, or stomach pain) that persist or recur, or if you have ulcers or bleeding problems unless directed by a doctor
Ask a doctor before use if you have
• frequent, burning urination for the first time • the stomach bleeding warning applying to you
• history of stomach problems, such as heartburn
• high blood pressure • heart disease • liver cirrhosis • bleeding problems
• diuretic use • ulcers • kidney disease • reached age 60 or older
Ask a doctor or pharmacist before use if you are
• taking any other drug containing an NSAID (prescription or nonprescription)
• taking a blood thinning (anticoagulant), steroid, diabetes, gout or arthritis drug
When using this product • do not take more than the recommended dosage
Stop and ask a doctor if
• product has been used for 3 days
• you experience any of the following signs of stomach bleeding:
• feel faint, vomit blood • have bloody or black stools • have stomach pain that does not get better
• ringing in the ears or a loss of hearing occurs
If pregnant or breast feeding, ask a health professional before use.
Directions: Adults and children 12 years and over: take 2 tablets with a full glass of water 3 times a day. Drink plenty of fluids. Children under 12 years: ask a doctor
Inactive ingredients:
benzoic acid, croscarmellose sodium, fd&c red #40, fd&c yellow #6, hypromellose, magnesium stearate, methacrylic acid-ethyl acrylate copolymer, microcrystalline cellulose, silicon dioxide, stearic acid, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triacetin, triethyl citrate
Other Information: • each tablet contains 25 mg of sodium • store at 59-86°F (15-30°C) in a dry place • protect from light • Tamper evident: tablets sealed in blisters. Do not use if blister foil or seal is open or damaged.