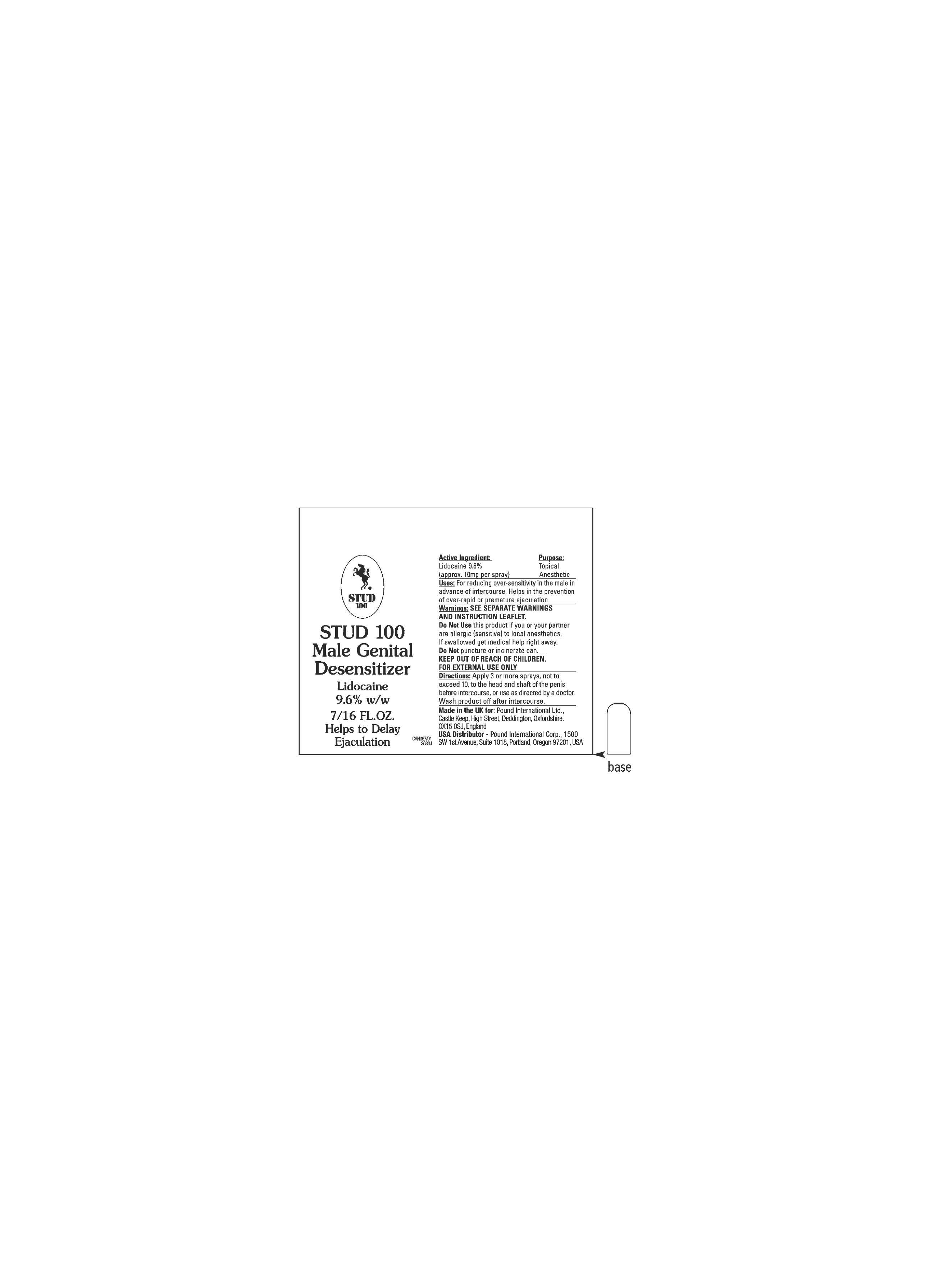
Active Ingredient Purpose
Lidocaine Base 9.6% w/w (approximately 10 mg per spray) Topical Anesthetic
Use
temporarily reduces sensitivity of the penis, which helps to delay ejaculation in cases of over-rapid or premature ejaculation (coming to a climax too quickly)
Keep out of reach of children. If swallowed get medical help or contact a Poison Control Center right away.
Stop and consult a doctor
if you or your partner develop a rash or irritation, such as burning or itching
if you do feel unwell or have any unpleasant effects after using the spray
If this product, used as directed, does not provide relief. Premature ejaculation may be due to a condition requiring medical supervision
Warnings
For external use only
Allergy alert: do not use this product if you or your partner are allergic (sensitive) to local Anesthetics.
Do not use - on broken or inflamed skin -if your partner is pregnant
Ask a doctor before use if you have, or ever had, liver or kidney problems
Ask a doctor or pharmacist before use if you are already taking prescribed drugs
when using this product
- do not get into eyes
- do not inhale
- do not exceed 24 sprays in 24 hours
Directions
-apply 3 or more sprays, not to exceed 10, to the head and shaft of the penis before intercourse, or as directed by a doctor
-wash product after intercourse
-correct quantity and time of application will be determined by individual requirements and you should always use the minimum effective quantity