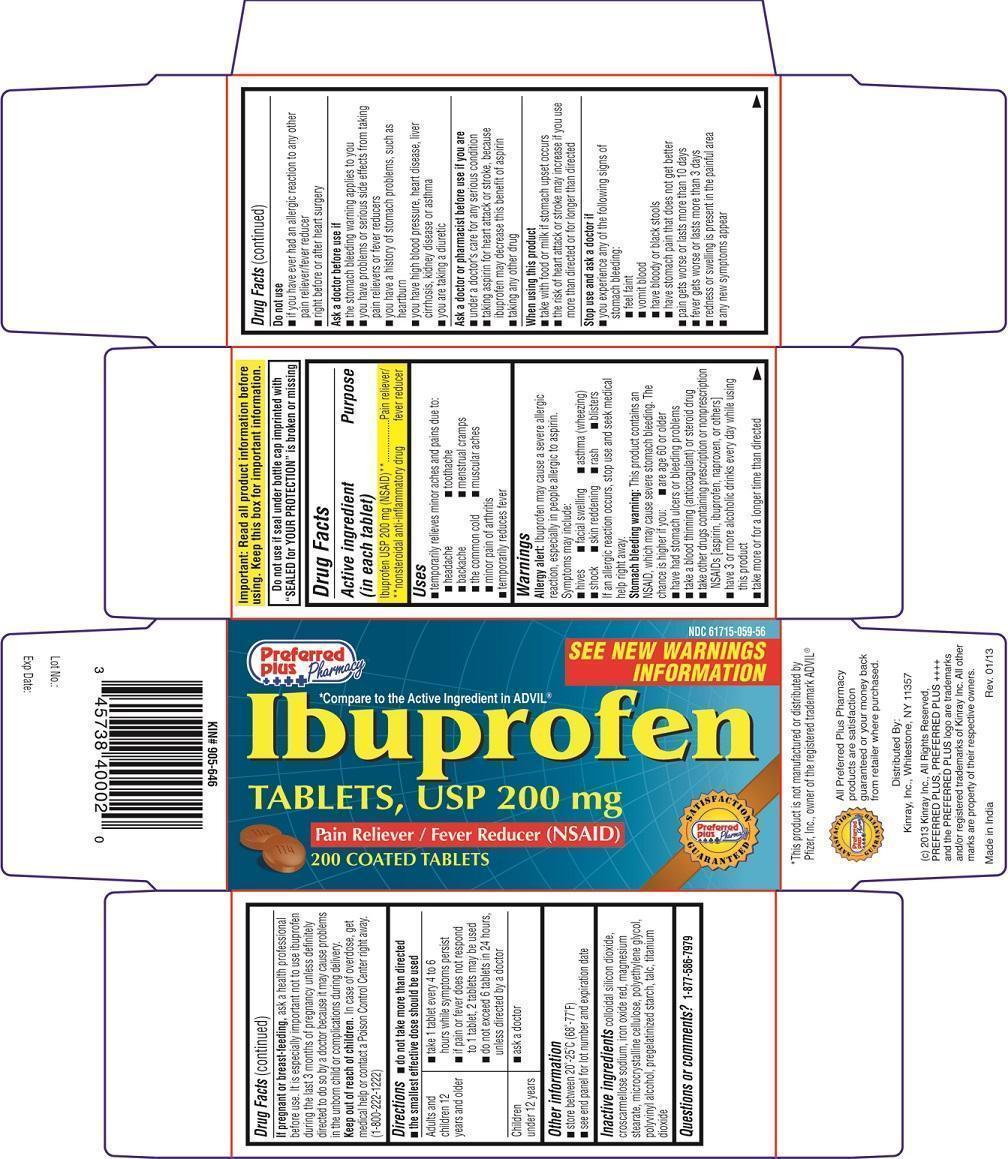
Active ingredient (in each tablet)
Ibuprofen USP 200 mg (NSAID)**
**nonsteroidal anti-inflammatory drug
Uses
- temporarily relieves minor aches and pain due to:
- headache
- toothache
- backache
- menstrual cramps
- the common cold
- muscular aches
- minor pain of arthritis
- temporarily reduces fever
Warnings
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks everyday while using this product
- take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/ fever reducer
- right before or after heart surgery
Ask a doctor before use if
- the stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease or asthma
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- taking any other drug
When using this product
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
Stop use and ask a doctor if
you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
If pregnant or breast-feeding
ask a health professional before use. It is especially important not to use ibuprofen during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- do not take more than directed
- the smallest effective dose should be used
| Adults and children 12 years and older |
|
| Children under 12 years |
|
Other Information
- store between 20o- 25oC (68o- 77oF)
- see end panel for lot number and expiration date
Inactive Ingredients:
colloidal silicon dioxide, croscarmellose sodium, iron oxide red, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, pregelatinized starch, talc, titanium dioxide
PRINCIPAL DISPLAY PANEL - 200 mg TABLET CARTON
Preferred Plus Pharmacy
NDC 61715-059-56
SEE NEW WARNINGS INFORMATION
*Compare to the Active Ingredient in ADVIL®
Ibuprofen TABLETS, USP 200 mg
Pain Reliever/Fever Reducer (NSAID)
200 COATED TABLETS
Distributed By:
Kinray, Inc., Whitestone, NY 11357
(c) 2013 Kinray Inc., All Rights Reserved, PREFERRED PLUS, PREFERRED PLUS ++++ and the PREFERRED PLUS logo are trademarks and/or registered trademarks of Kinray Inc. All other marks are property of their respective owners.