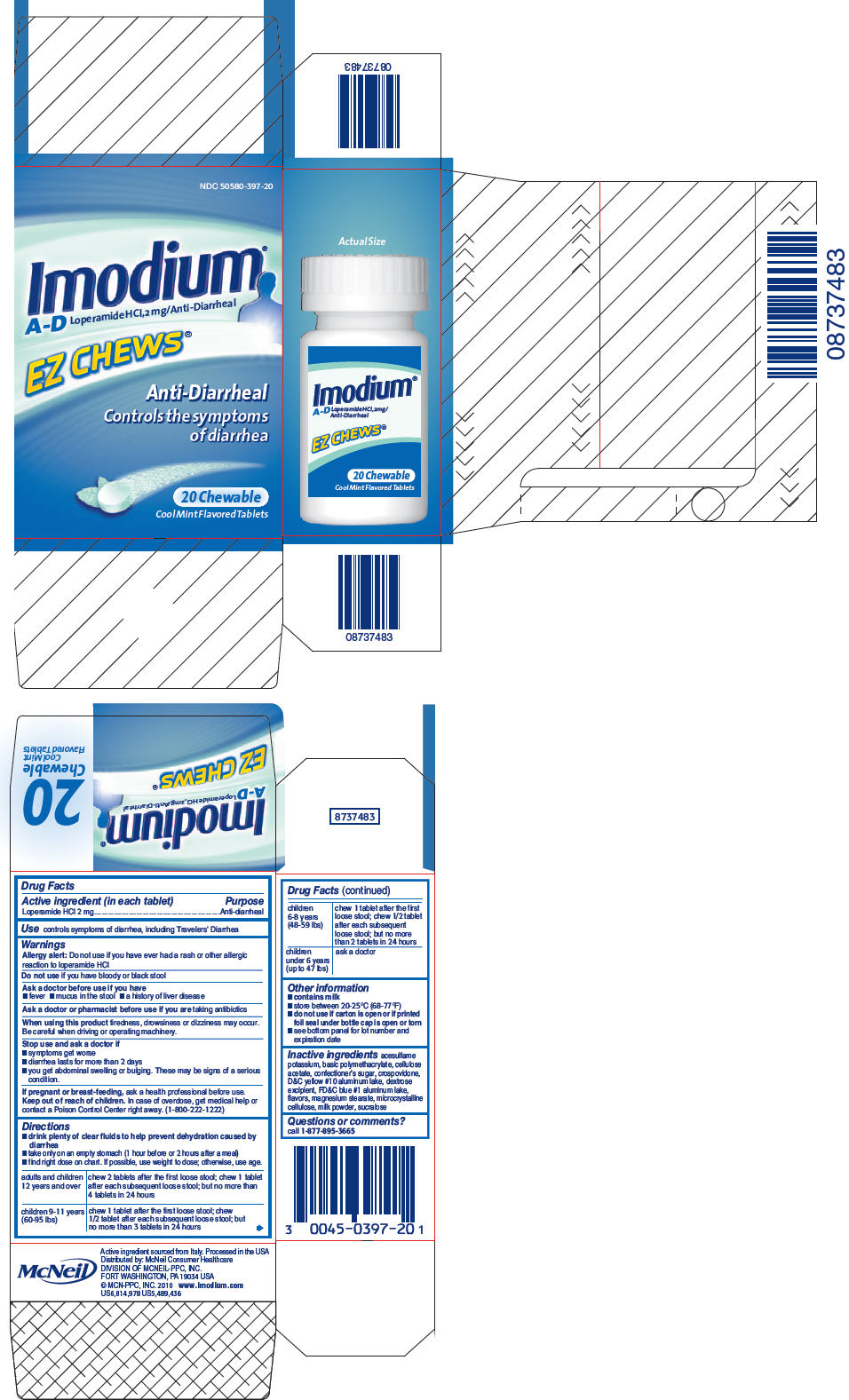
IMODIUM A-D- loperamide hydrochloride tablet, chewable
McNeil Consumer Healthcare Div. McNeil-PPC, Inc
----------
Imodium®
A-D
Warnings
When using this product tiredness, drowsiness or dizziness may occur. Be careful when driving or operating machinery.
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- take only on an empty stomach (1 hour before or 2 hours after a meal)
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
| adults and children 12 years and over | chew 2 tablets after the first loose stool; chew 1 tablet after each subsequent loose stool; but no more than 4 tablets in 24 hours |
| children 9-11 years (60-95 lbs) | chew 1 tablet after the first loose stool; chew ½ tablet after each subsequent loose stool; but no more than 3 tablets in 24 hours |
| children 6-8 years (48-59 lbs) | chew 1 tablet after the first loose stool; chew ½ tablet after each subsequent loose stool; but no more than 2 tablets in 24 hours |
| children under 6 years (up to 47 lbs) | ask a doctor |
Other information
- contains milk
- store between 20-25° C (68-77° F)
- do not use if carton is open or if printed foil seal under bottle cap is open or torn
- see bottom panel for lot number and expiration date
| IMODIUM
A-D
loperamide hydrochloride tablet, chewable |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - McNeil Consumer Healthcare Div. McNeil-PPC, Inc (878046358) |
Revised: 1/2015
Document Id: 164b2894-1195-40e6-b324-b12567291085
Set id: d011ae1a-680c-4476-a489-5407df13648f
Version: 3
Effective Time: 20150129
McNeil Consumer Healthcare Div. McNeil-PPC, Inc