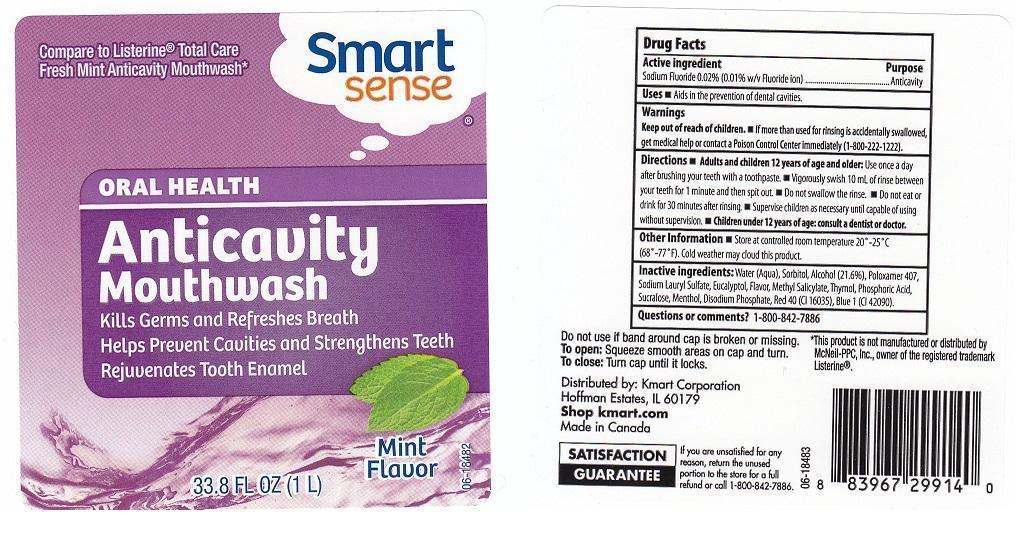
SMART SENSE ANTICAVITY- sodium fluoride liquid
KMART CORPORATION
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
SODIUM FLUORIDE 0.02% (0.01% W/V FLUORIDE ION)
USES
AIDS IN THE PREVENTION OF DENTAL CAVITIES.
WARNINGS
KEEP OUT OF REACH OF CHILDREN. IF MORE THAN USED FOR RINSING IS ACCIDENTALLY SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY (1-800-222-1222).
KEEP OUT OF REACH OF CHILDREN
IF MORE THAN USED FOR RINSING IS ACCIDENTALLY SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER IMMEDIATELY (1-800-222-1222).
DIRECTIONS
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER: USE ONCE A DAY AFTER BRUSHING YOUR TEETH WITH A TOOTHPASTE. VIGOROUSLY SWISH 10 ML OF RINSE BETWEEN YOUR TEETH FOR 1 MINUTE AND THEN SPIT OUT. DO NOT SWALLOW THE RINSE. DO NOT EAT OR DRINK FOR 30 MINUTES AFTER RINSING. SUPERVISE CHILDREN AS NECESSARY UNTIL CAPABLE OF USING WITHOUT SUPERVISION. CHILDREN UNDER 12 YEARS OF AGE: CONSULT A DENTIST OR DOCTOR.
OTHER INFORMATION
STORE AT CONTROLLED ROOM TEMPERATURE 20-25C (68-77F). COLD WEATHER MAY CLOUD THIS PRODUCT.
INACTIVE INGREDIENTS:
WATER (AQUA), SORBITOL, ALCOHOL (21.6%), POLOXAMER 407, SODIUM LAURYL SULFATE, EUCALYPTOL, FLAVOR, METHYL SALICYLATE, THYMOL, PHOSPHORIC ACID, SUCRALOSE, MENTHOL, DISODIUM PHOSPHATE, RED 40 (CI 16035), BLUE 1 (CI 42090).
QUESTIONS OR COMMENTS?
1-800-842-7886
LABEL COPY