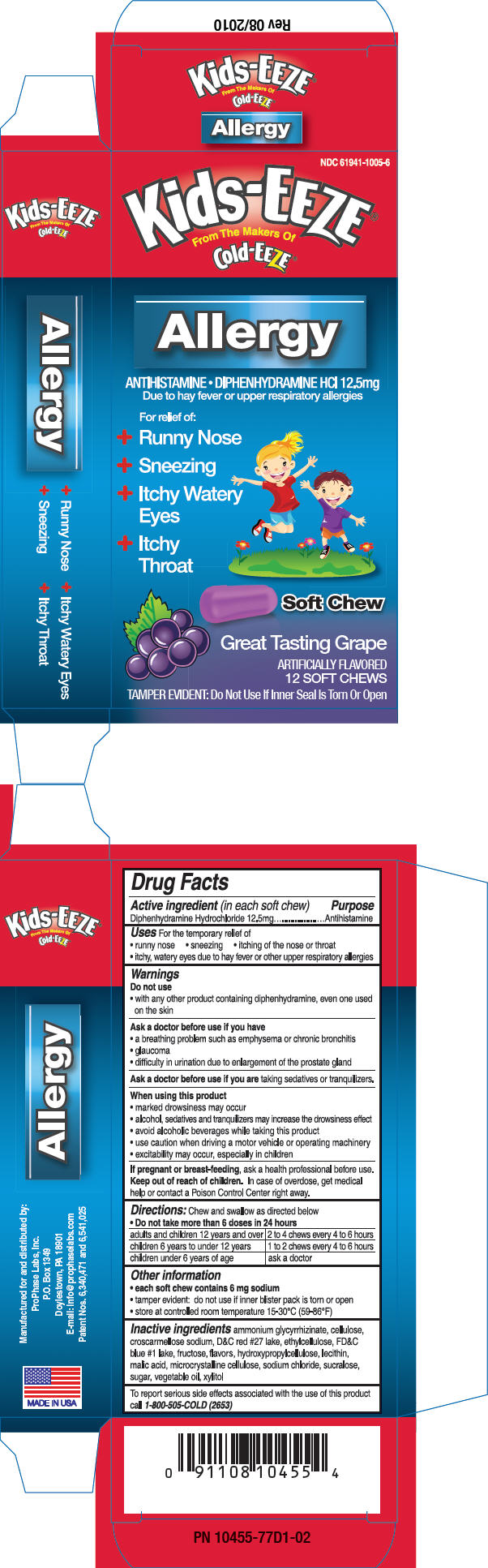
Uses
For the temporary relief of
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes due to hay fever or other upper respiratory allergies
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
Chew and swallow as directed below
- Do not take more than 6 doses in 24 hours
| adults and children 12 years and over | 2 to 4 chews every 4 to 6 hours |
| children 6 years to under 12 years | 1 to 2 chews every 4 to 6 hours |
| children under 6 years of age | ask a doctor |
Other information
- each soft chew contains 6 mg sodium
- tamper evident: do not use if inner blister pack is torn or open
- store at controlled room temperature 15-30°C (59-86°F)
Inactive ingredients
ammonium glycyrrhizinate, cellulose, croscarmellose sodium, D&C red #27 lake, ethylcellulose, FD&C blue #1 lake, fructose, flavors, hydroxypropylcellulose, lecithin, malic acid, microcrystalline cellulose, sodium chloride, sucralose, sugar, vegetable oil, xylitol
PRINCIPAL DISPLAY PANEL - 12.5 mg Package
NDC 61941-1005-6
Kids-EEZE®
From The Makers Of
Cold-EEZE®
Allergy
ANTIHISTAMINE • DIPHENHYDRAMINE HCl 12.5mg
Due to hay fever or upper respiratory allergies
For relief of:
- +
- Runny Nose
- +
- Sneezing
- +
- Itchy Watery
Eyes - +
- Itchy
Throat
Soft Chew
Great Tasting Grape
ARTIFICIALLY FLAVORED
12 SOFT CHEWS
TAMPER EVIDENT: Do Not Use If Inner Seal Is Torn Or Open