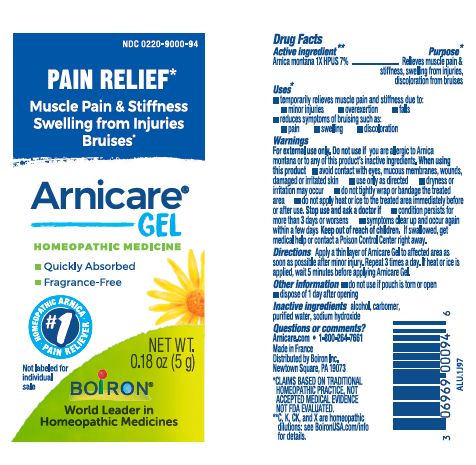
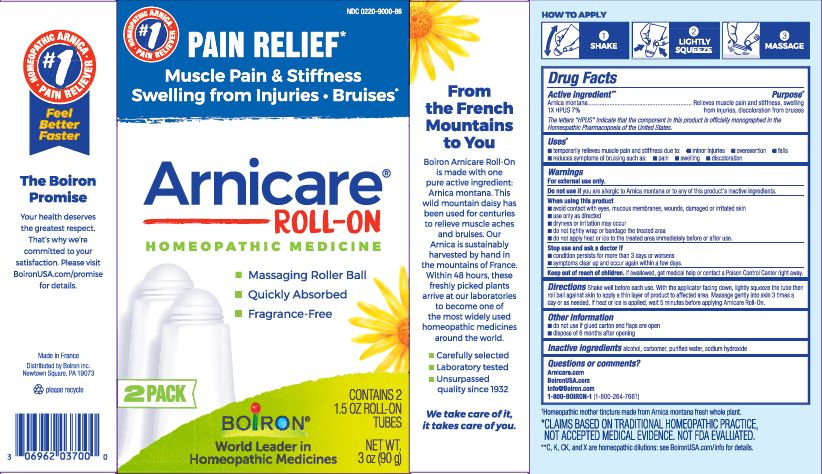
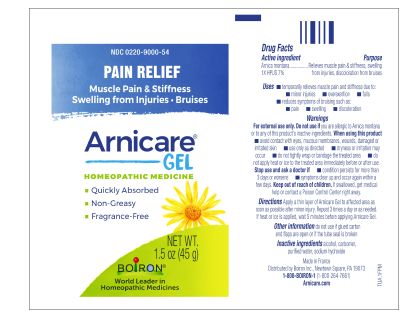
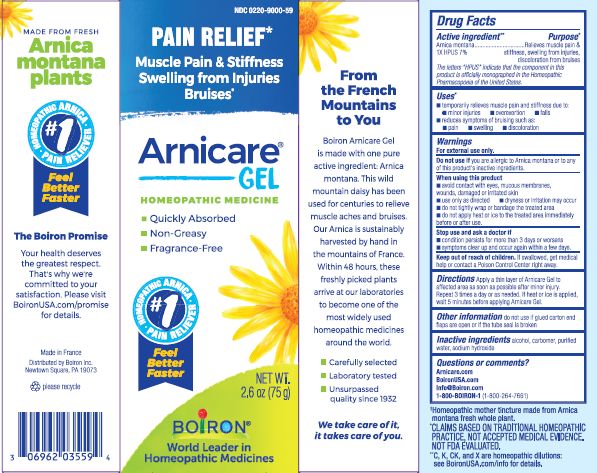
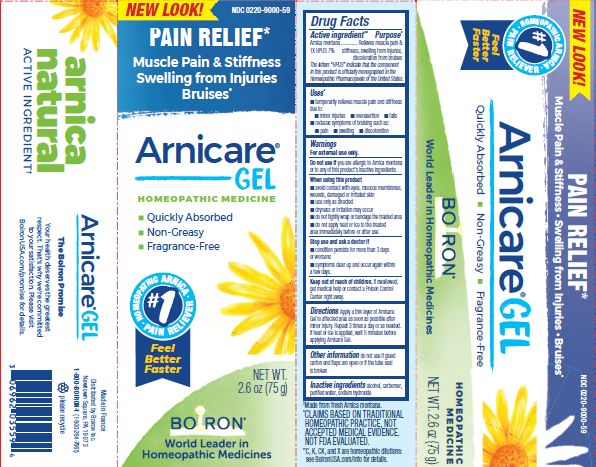
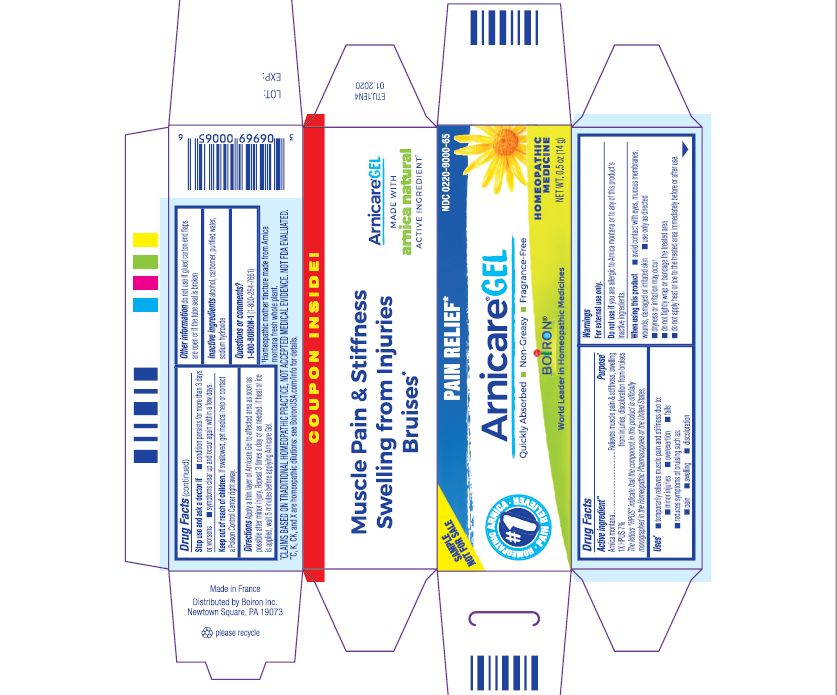
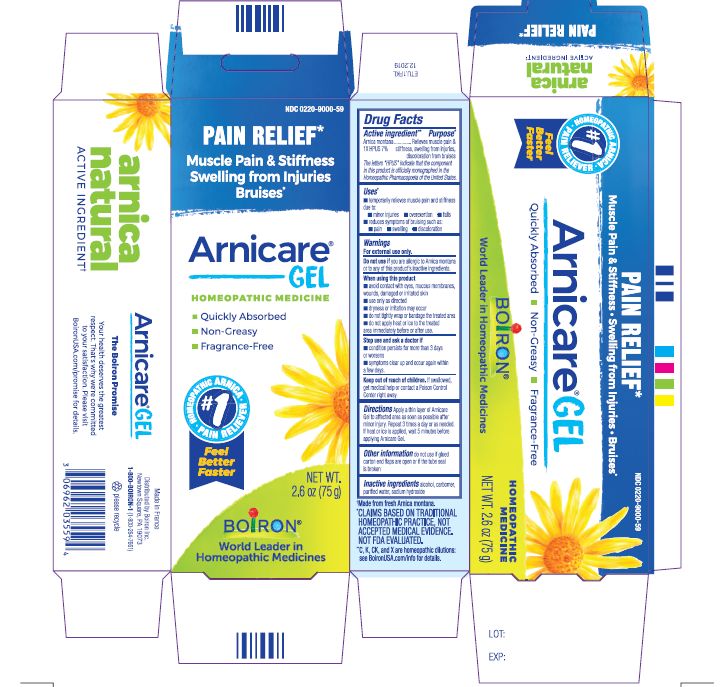
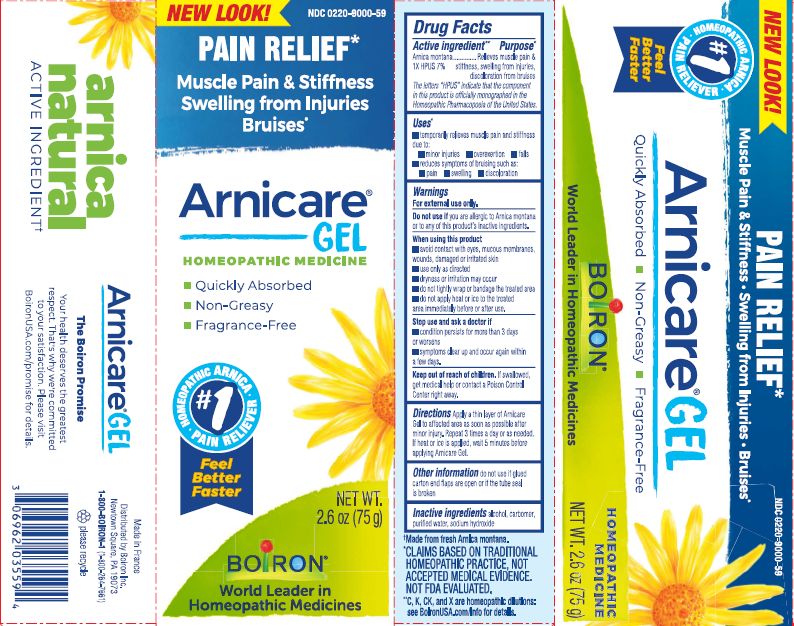
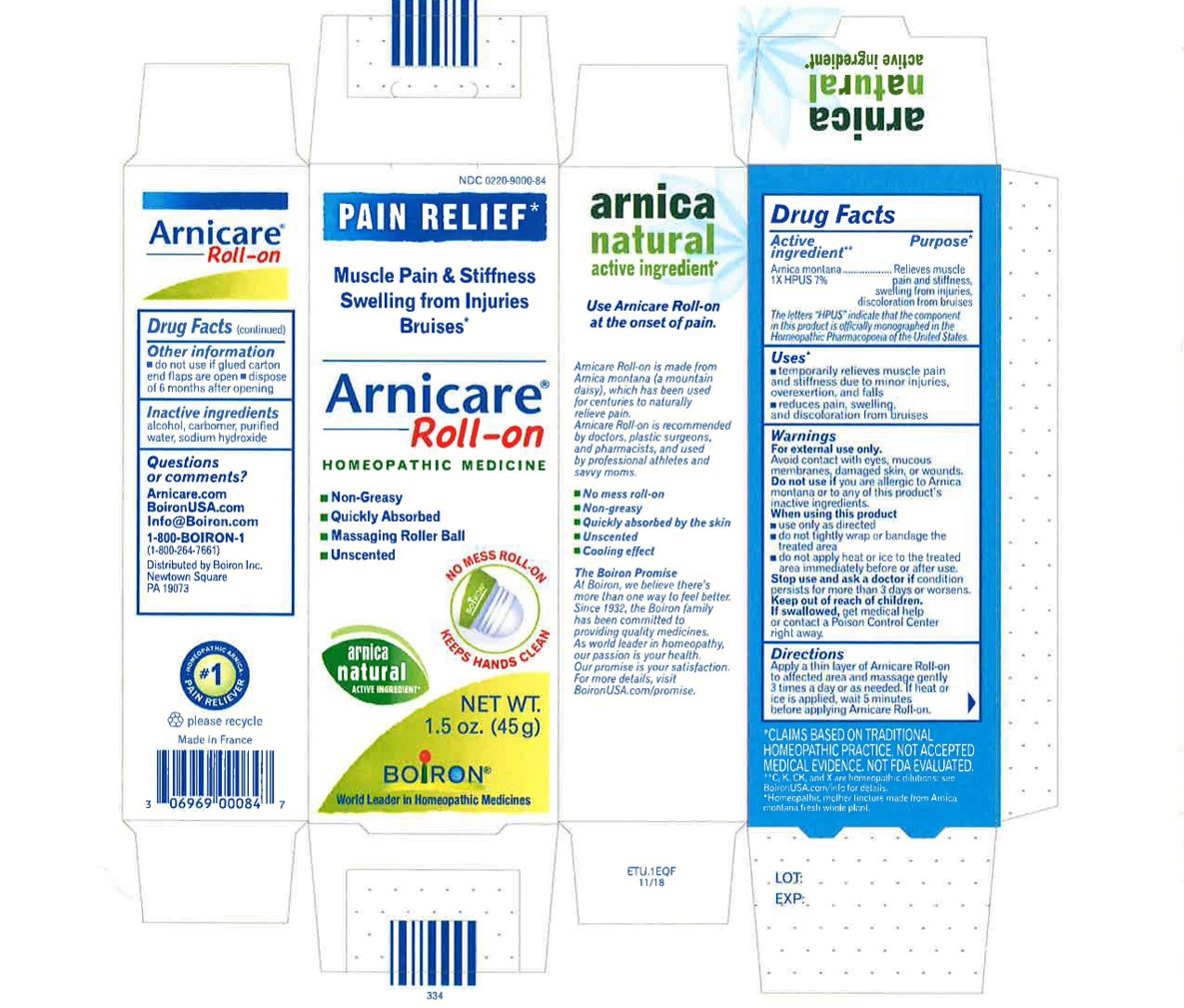
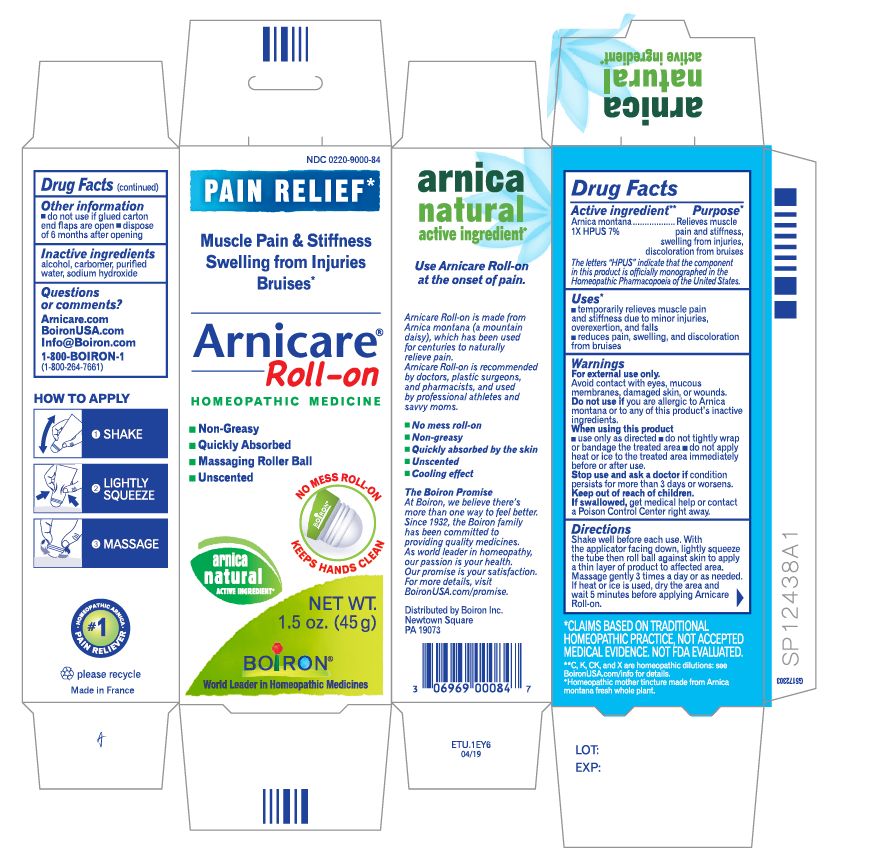
Active ingredient**
Arnica montana 1X HPUS 7%
The letters HPUS indicate that the component in this product is officially monographed in the Homeopathic Pharmacopoeia of the United States.
Purpose*
Arnica montana 1X HPUS 7% Relieves muscle pain & stiffness, swelling from injuries, discoloration from bruises
Uses*
temporarily relieves muscle pain and stiffness due to:
- minor injuries
- overexertion
- falls
reduces symptoms of bruising such as:
- pain
- swelling
- discoloration
Warnings
For external use only.
Do not use if you are allergic to Arnica montana or to any of this product's inactive ingredients.
When using this product
avoid contact with eyes, mucous membranes, wounds, damaged or irritated skin
use only as directed
dryness or irritation may occur
do not tightly wrap or bandage the treated area
do not apply heat or ice to treated area immediately before or after use
Stop use and ask a doctor if
- condition persists for more than 3 days or worsens
- symptoms clear up and occur again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
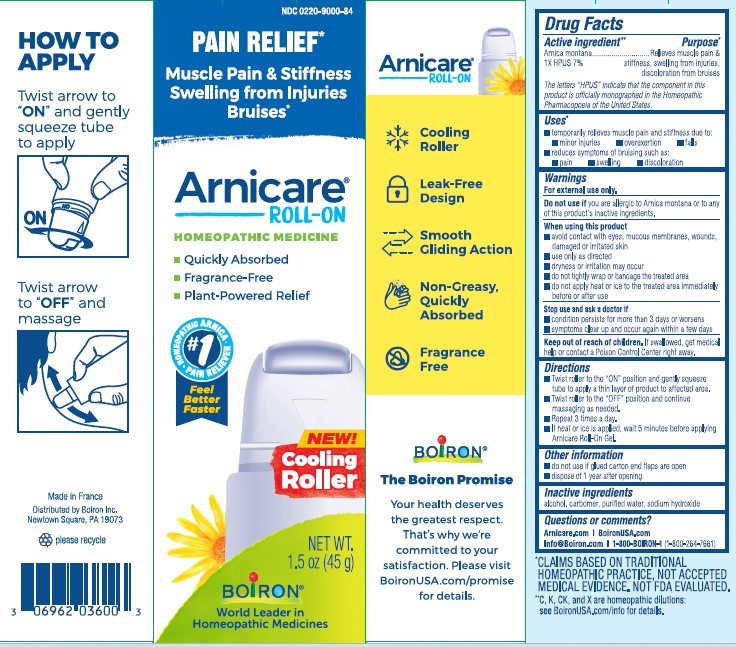
Directions
Apply a thin layer of Arnicare Gel to the affected area as soon as possible after minor injury. Repeat 3 times a day or as needed. If heat or ice is applied, wait 5 minutes before applying Arnicare Gel.
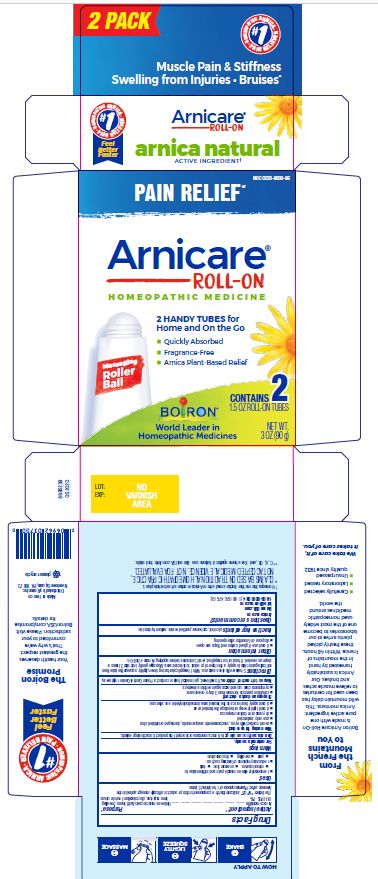
Arnicare Gel Roll-On (2019)- Shake well before each use. With the applicator facing down, lightly squeeze the tube then roll ball against skin to apply a thin layer of the product to affected area. Massage gently into skin 3 times a day or as needed. If heat or ice is applied, wait 5 minutes before applying Arnicare Roll-on.
Arnicare Gel Roll-On (2023)- Twist roller to the "ON" position and gently squeeze tube to apply a thin layer of product to affected area. Twist roller to the "OFF" position and continue massaging as needed. Repeat 3 times a day. If heat or ice is applied, wait 5 minutes before applying Arnicare Roll-on Gel.
Other information
do not use if glued carton end flaps are open or if the tube seal is broken
do not use if pouch is torn or open
Arnicare Gel 0.18 oz (5g)- dispose 1 day after opening
Arnicare Gel Roll-On (2019)- dispose of 6 months after opening
Arnicare Gel Roll-On (2023)- dispose of 1 year after opening
0.18 oz (5g)
0.5 oz (14g)
1.5 oz (45g)
2.6 oz (75g)
4.2 oz (120g)
3 oz (90g)
Pain Relief*
Muscle Pain & Stiffness Swelling from Injuries Bruises*
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
*C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.