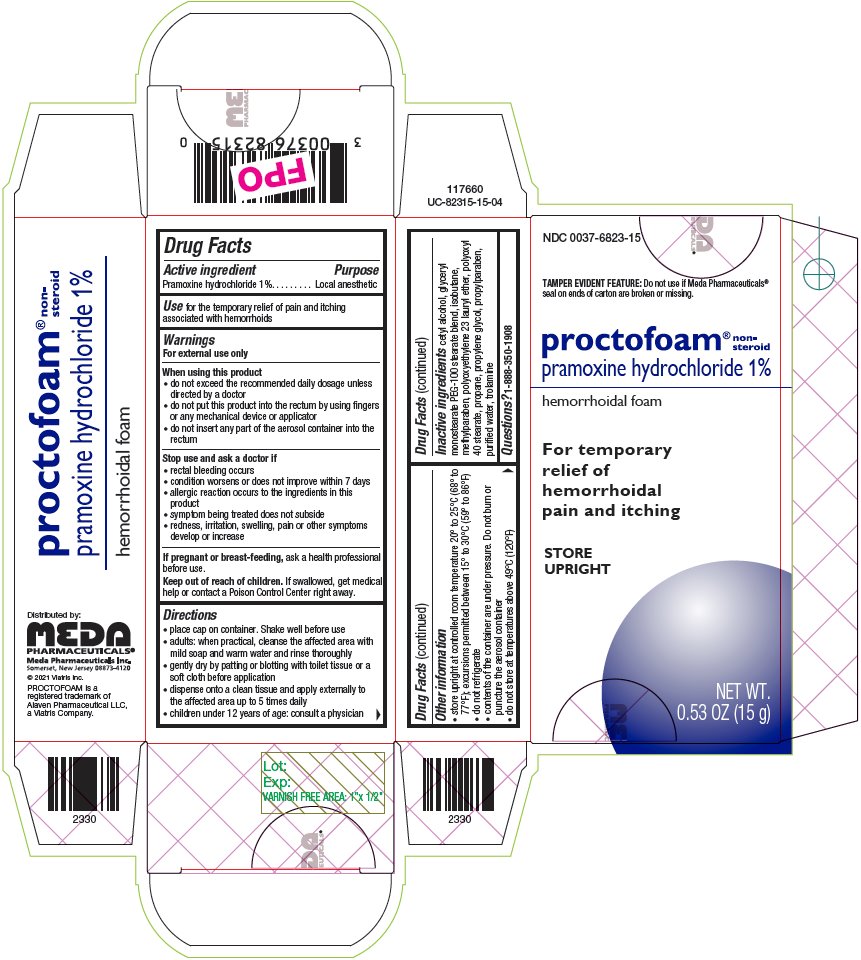
Warnings
For external use only
When using this product
- •
- do not exceed the recommended daily dosage unless directed by a doctor
- •
- do not put this product into the rectum by using fingers or any mechanical device or applicator
- •
- do not insert any part of the aerosol container into the rectum
Directions
- •
- place cap on container. Shake well before use
- •
- adults: when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
- •
- gently dry by patting or blotting with toilet tissue or a soft cloth before application
- •
- dispense onto a clean tissue and apply externally to the affected area up to 5 times daily
- •
- children under 12 years of age: consult a physician
Other information
- •
- store upright at controlled room temperature 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F)
- •
- do not refrigerate
- •
- contents of the container are under pressure. Do not burn or puncture the aerosol container
- •
- do not store at temperatures above 49°C (120°F)
Inactive ingredients
cetyl alcohol, glyceryl monostearate PEG-100 stearate blend, isobutane, methylparaben, polyoxyethylene 23 lauryl ether, polyoxyl 40 stearate, propane, propylene glycol, propylparaben, purified water, trolamine
Questions?
1-888-350-1908
Distributed by:
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120
© 2021 Viatris Inc.
PROCTOFOAM is a registered trademark of Alaven Pharmaceutical LLC, a Viatris Company..
Principal Display Panel – 1%
NDC 0037-6823-15
TAMPER EVIDENT FEATURE: Do not use if Meda Pharmaceuticals® seal on ends of carton are broken or missing.
proctofoam® non-steroid
pramoxine hydrochloride 1%
hemorrhoidal foam
For temporary
relief of
hemorrhoidal
pain and itching
STORE
UPRIGHT
NET WT.
0.53 OZ (15 g)