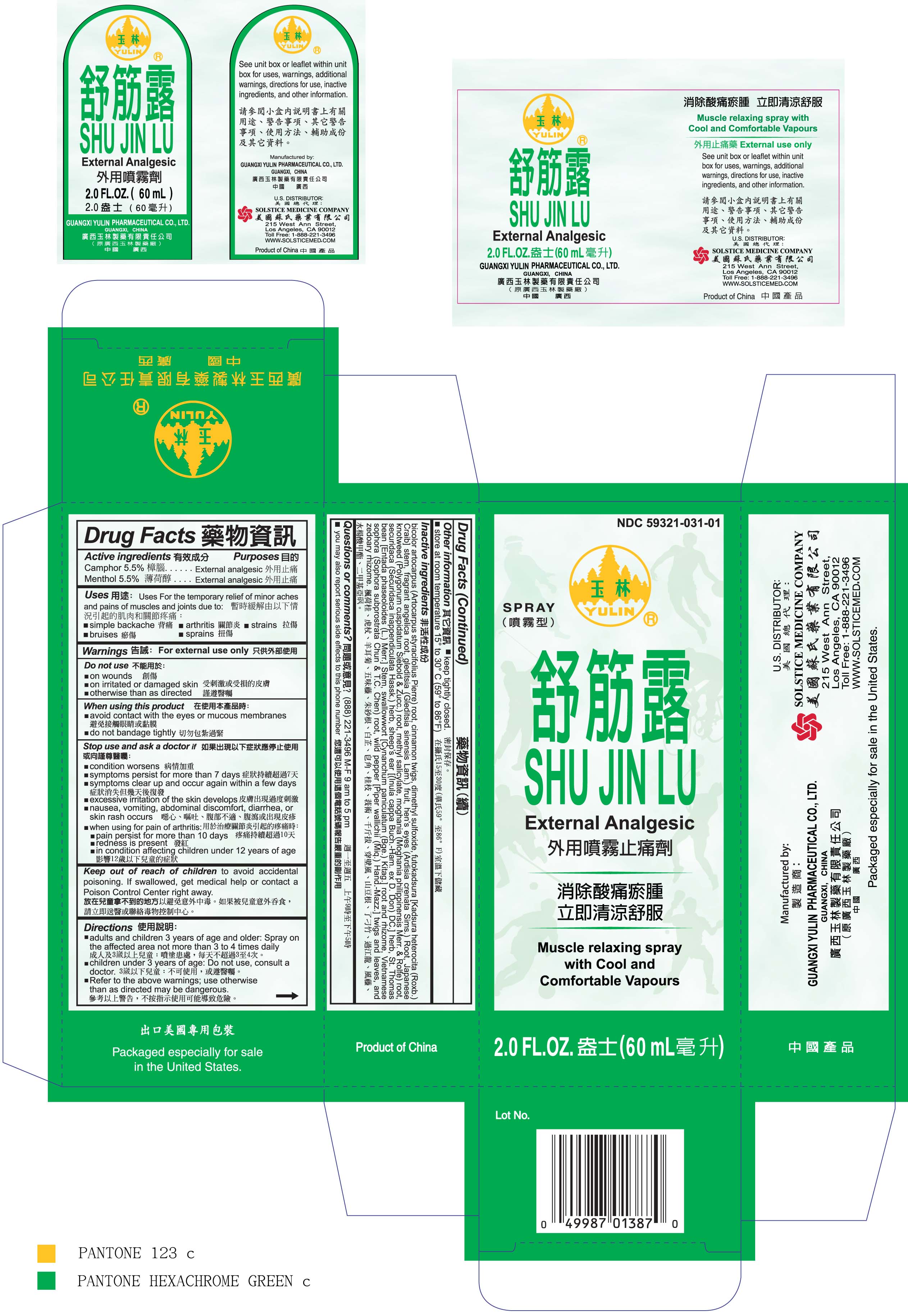
Uses
For the temporary relief of minor aches and pains of muscles and joints due to:
simple backache
arthritis
strains
bruises
sprains
Stop use and ask a doctor if
condition worsens
symptoms persist for more than 7 days
symptoms clear up and occur again within a few days
excessive irritation of the skin develops
nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
when using for pain of arthritis:
pain persists for more than 10 days
redness is present
in conditions affecting children under 12 years of age
Keep out of reach of children to avoid accidental poisoning.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
adults and children 3 years of age and older: Spray on the affected area not more than3 to 4 times daily
children under 3 years of age: Do not use, consult a doctor
Refer to the above warnings; use otherwise than as directed may be dangerous.
Inactive ingredients
bicolor artocarpus (Artocarpus styracifolius Pierre) root,
cinnamon twigs,
dimethyl sulfoxide,
futokadsura Kadsura heterocita (Roxb.) Craib stem,
fragrant angelica root,
gleditsia (Gleditsia sinensis Lam.) fruit,
hen’s eyes (Ardisia crenata Sims.) Root,
Japanese knotweed (Polygonum cuspidatum Siebold Zucc.) root,
methyl salicylate,
moghania (Moghania philippinensis Merr. Rolfe) root,
securidaca (Securidaca inappendiculata Hassk.) herb,
sheep’s ear (Inula cappa Buch.-Ham. ex D. Don) DC. herb,
St. Thomas bean Entada phaseoloides (L.) Merr. Stem,
swallowwort Cynanchum paniculatum (Bge.) Kitag. root and rhizome,
Vietnamese sophora (Sophora subprostrata Chun T.C. Chen) root,
wild pepper Piper wallichii (Miq.) Hand.-Mazz. twigs and leaves,
and zedoary rhizome.