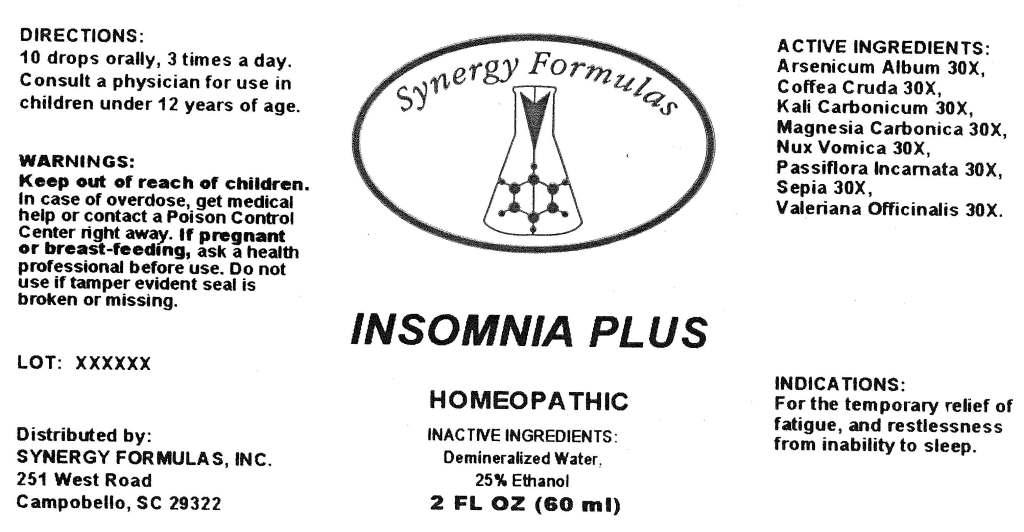
INSOMNIA PLUS - arsenicum album, coffea cruda, kali carbonicum, magnesia carbonica, nux vomica, passiflora incarnata, sepia, valeriana officinalis, liquid
Apotheca Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS: Arsenicum album 30X, Coffea cruda 30X, Kali carbonicum 30X, Magnesia carbonica 30X, Nux vomica 30X, Passiflora incarnata 30X, Sepia 30X, Valeriana officinalis 30X.
INDICATIONS: For the temporary relief of fatigue, and restlessness from inability to sleep.
WARNINGS: Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
If pregnant or breast-feeding, ask a health professional before use.
Do not use if tamper evident seal is broken or missing.
DIRECTIONS: 10 drops orally, 3 times a day. Consult a physician for use in children under 12 years of age.
INACTIVE INGREDIENTS: Demineralized water, 25% Ethanol.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or contact a Poison Control Center right away.
INDICATIONS: For the temporary relief of fatigue, and restlessness from inability to sleep.
Distributed by:
SYNERGY FORMULAS, INC.
251 West Road
Campobello, SC 29322
Synergy Formulas
INSOMNIA PLUS
HOMEOPATHIC
2 FL OZ (60 ml)