DOSAGE AND ADMINISTRATION
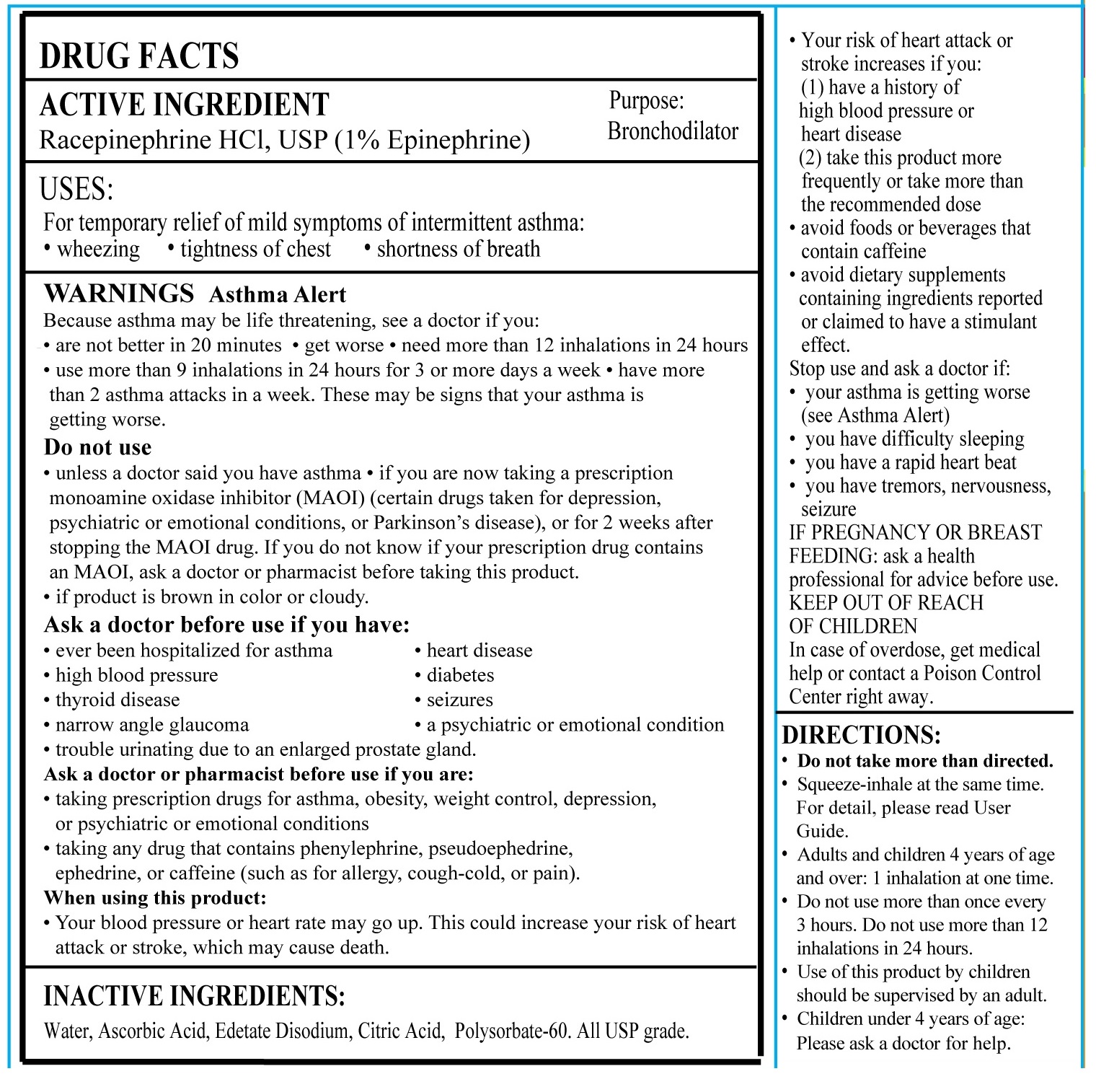
DIRECTIONS
• Do not take more than directed
• Place your lips around the mouthpiece and squeeze the bulb while inhaling simultaneously to breathe in the epinephrine aerosol– see figure below for details
• Adults and children 4 years of age and over: Do not take more than one dose every 3 hours. Do not take more than 8 doses in a 24-hour period. The use of this product by children under the age of 12 should be supervised by an adult.
• For children’s usage between 4 to 12 years of age: an adult may squeeze the bulb while your child inhales.
• Please contact your doctor if your symptoms advance.
How Supplied
HOW SUPPLIED
Each unit of EpiMist contains 10 ml, 250 doses of epinephrine. Each dose (40 microliters) is delivered by one (1) turn-click of the device, containing 1% epinephrine solution made with racepinephrine hydrogen chloride, USP.
Storage and Handling
STORAGE AND HANDLING
1) Protect from light to reduce loss of the activity of the active ingredient.
2) Avoid excessive heat.
3) You can carry the whole set during daytime but avoid excessive heat and bright light.
4) When at home, it is recommended to store in refrigerator (35 to 40 oF).
5) After the liquid medicine is used out, dispose the plastic device properly.
Inactive Ingredient Section
INACTIVE INGREDIENT
Water, Edetate Disodium, Ascorbic Acid, Citric Acid, Polysobate-60. All USP grade.