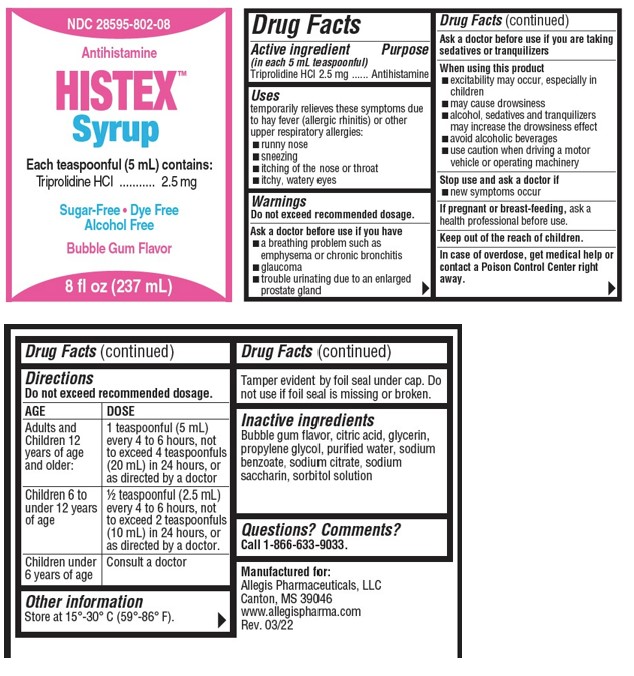
Uses
temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
Warnings
Do not exceed recommended dosage.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor before use if you are taking sedatives or tranquilizers
Directions
Do not exceed recommended dosage.
| AGE | DOSE |
|---|---|
| Adults and Childen 12 years of age and older: | 1 teaspoonful (5 mL) every 4 to 6 hours, not to exceed 4 teaspoonfuls (20 mL) in 24 hours, or as directed by a doctor |
| Childen 6 to under 12 years of age | ½ teaspoonful (2.5 mL) every 4 to 6 hours, not to exceed 2 teaspoonfuls (10 mL) in 24 hours, or as directed by a doctor. |
| Children under 6 years of age | Consult a doctor |
Other Information
Store at 15°-30° C (59°-86° F).
Tamper evident by foil seal under cap. Do not use if foil seal is missing or broken.
Dispense in a tight, light-resistant container with a child-resistant cap.