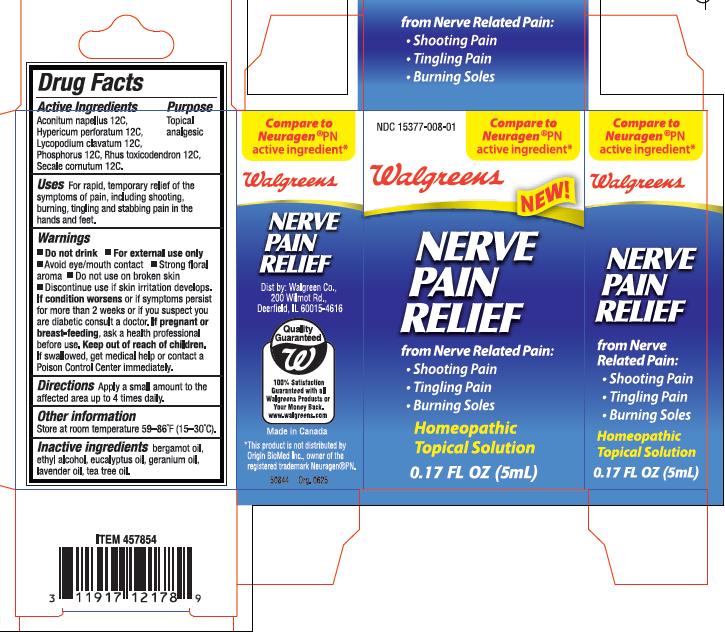
WALGREENS NERVE PAIN RELIEF - aconitum napellus, hypericum perforatum, lycopodium clavatum, phosphorus, rhus toxicodendron, secale cornutum solution
Origin Biomed Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Uses: For rapid, temporary relief of the symptoms of pain, including shooting, burning, tingling and stabbing pains in the hand and feet.
- Do not drink
- For external use only
- Avoid eye/mouth contact
- Strong floral aroma
- Do not use on broken skin
Discontinue use if skin irritation develops.
If condition worsens or if symptoms persist for more than 2 weeks or if you suspect you are diabetic consult a doctor.
If pregnant or breast feeding, ask a health care professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions: Apply a small amount to the affected area up to 4 times daily
Store at room temperature 59-86 degrees F (15-30 degrees C)
Nerve Pain Relief
from Nerve Related Pain:
- Shooting Pain
- Tingling Pain
- Burning Soles
Aconitum napellus 12C, Hypericum perforatum 12C, Lycopodium clavatum 12C, Phosphorus 12C, Rhus toxicodendron 12C, Secale Cornatum 12C
bergamot oil, ethyl alcohol, eucalyptus oil, geranium oil, lavender oil, tea tree oil
Origin Biomed Inc.